Abstract
Several lines of evidence support the premise that screening colonoscopy reduces colorectal cancer (CRC) incidence, but there may be differential benefits for right- and left-sided tumors. To better understand the biological basis of this differential effect, we derived bio-mathematical models of CRC incidence trends in US and UK populations, representing relatively high- and low-prevalent screening, respectively.
Using the Surveillance Epidemiology and End Results (SEER) and the Office of National Statistics (ONS) registries (both 1973 to 2006), we derived stochastic multistage clonal expansion (MSCE) models for right- (proximal colon) and left- (distal colon and rectal) sided tumors. The MSCE concept is based on the initiation-promotion-progression paradigm of carcinogenesis and provides a quantitative description of natural tumor development from the initiation of an adenoma (via biallelic tumor suppressor gene inactivation) to the clinical detection of CRC.
From 1,228,036 (SEER:340,582; ONS:887,454) cases, parameter estimates for models adjusted for calendar-year and birth-cohort effects showed that adenoma initiation rates were higher for right-sided tumors while paradoxically adenoma growth rates were higher for left-sided tumors. The net effect was a higher cancer risk in the right colon after age 70 years. Consistent with this finding, simulations of adenoma development predicted that the relative prevalence for right- versus left-sided tumors increases with increasing age; a differential effect most striking in women.
Interpretation: Using a realistic bio-mathematical description of CRC development for two nationally representative registries, we demonstrate age- and sex-dependent biological gradients for right- and left-sided colorectal tumors. These findings argue for an age-, sex- and site-directed approach to CRC screening.
Keywords: colorectal cancer, screening, bio-mathematical modeling, incidence trends, colorectal adenoma
Introduction
Colorectal cancer (CRC) is the second commonest cause of cancer deaths in the United States (US)(1) and United Kingdom (UK)(2). Screening for cancer and its benign precursor (adenoma) offers the best opportunities to reduce cancer-related mortality. The American Cancer Society, the American College of Radiology, and the US Multi-Society Task Force on Colorectal Cancer guidance on screening recommends a cancer ‘prevention’, rather than cancer ‘detection’, approach - the former including screening colonoscopy (1). Although untested by randomized trials, there are several lines of evidence supporting the effectiveness of screening colonoscopy including: (i) reductions in CRC mortality in trials of fecal occult blood (FOB) screening(3); (ii) mathematical modeling (4); (iii) case-control studies(5, 6); and (iv) population-based studies following the initiation of large-scale colonoscopy programs(7). Accordingly, screening colonoscopy rates have increased three- to eight-fold over the past two decades in the US(8, 9). By contrast, in the UK, screening colonoscopy utilization rates are low and limited to high-risk patients, as FOB screening has been offered through a national-based program only since 2006(10).
Studies of secular trends of CRC incidences in the US consistently demonstrate declining incidence rates for left-sided cancers with barely any decreases for right-sided cancer incidences(11–13); changes which are generally attributed to the increasing utilization of endoscopic screening(11). Slower declines in left-sided cancer incidences in Blacks(13) and Asia-Pacific populations(12) compared with Whites that parallel the generally lower utilization of endoscopic screening among Blacks support this hypothesis(1). It is tempting to posit that the relative decline of left-sided cancers is due mainly to flexible sigmoidoscopy as historically utilization rates were higher than those for colonoscopy. However, the choice of screening endoscopic modality has substantially reversed in the past decade – for example, among Medicare fee-for-service beneficiaries, flexible sigmoidoscopy rates halved from a period in the late 1990s to 2003, while colonoscopy rates increased almost eight-fold(8) – yet the left-sided cancer incidence decline has remained the dominant effect. Furthermore, Baxter and colleagues(14), using a province-wide administrative database in Canada, recently reported that among CRC cases, previous exposure to complete colonoscopy was strongly associated with fewer deaths from left-sided CRC (odds ratio: 0.33) but not for right-sided CRC (OR: 0.99). This apparent lack of effectiveness for right-sided lesions with screening colonoscopy may be due to higher miss rates for right-sided cancers – although, the estimate for this misclassification is modest (2% to 6%)(15). In aggregate, these observations lead us and others(16) to hypothesize that the differential effects are, at least in part, due to biological differences between right- and left-sided disease.
In the absence of randomized trials to test this hypothesis, this study uses population-based CRC incidence data covering three decades to fit multistage clonal expansion (MSCE) models of CRC that were previously developed and published(17–19). The MSCE models capture the salient biological processes (including tumor initiation and promotion) involved in CRC and allow quantitative adjustments of the age-specific cancer incidence for calendar year and birth cohort effects. These effects capture indirectly the impact of screening and other environmental factors on CRC incidence. The direct comparison of two large cancer registries - the Surveillance Epidemiology and End Results (SEER) in the US and the Office of National Statistics (ONS) in the UK representing relatively high- and low-prevalent screening populations, respectively - strengthens our conclusions regarding the eminent role of tumor initiation and promotion in the apparent cancer risk differential between left-sided and right-sided colon.
Methods
Incidence Data
US incidence data for CRC were obtained from the SEER registry for the years 1973–2006. We extracted reported CRC cases by anatomic subsite, sex, race, age and calendar year in the nine SEER geographic areas, which together represent an estimated 9.5% of the U.S. population. The subsites were categorized using the International Classification of Diseases, 3rd Revision (ICD-O-3) codes as proximal colon: 180 through 184; distal colon: 185 through 187; and rectum: 199 and 209. Person-years by sex, race, age and calendar year were obtained from the SEER registry (based on U.S. Census Bureau). For comparability with the UK data, model fits were restricted to Whites (including Hispanics) by sex.
Data for CRC cases in England and Wales were obtained from the Office of National Statistics (ONS) for the years 1973–2006 (for the purpose of this analysis, combined England and Wales was taken as synonymous with UK). In the absence of a recoding system as exists for SEER, subsites were categorized into proximal and distal colon, and rectum using ICD-10 codes (equivalent to ICD-O-3 above) and equivalent subsite codes for ICD-8 and ICD-9 (supplemental material p4). As the proportion of colon cancers classified as “colon not otherwise specified (NOS)” in the UK dataset was substantial (up to 18%), we randomly recoded NOS cases proportionate to the subsite distribution at the corresponding age and year of diagnosis. The population bases by age, sex and calendar year were obtained from ONS population census data. For the analysis period, we assumed that the great majority of the UK population was White.
For the trends in incidence baseline analyses, data were standardized to the US 2000 total population. We used the terms ‘right-sided’ as equivalent to proximal colon and ‘left-sided’ as distal colon and rectum combined.
Bio-mathematical CRC Model
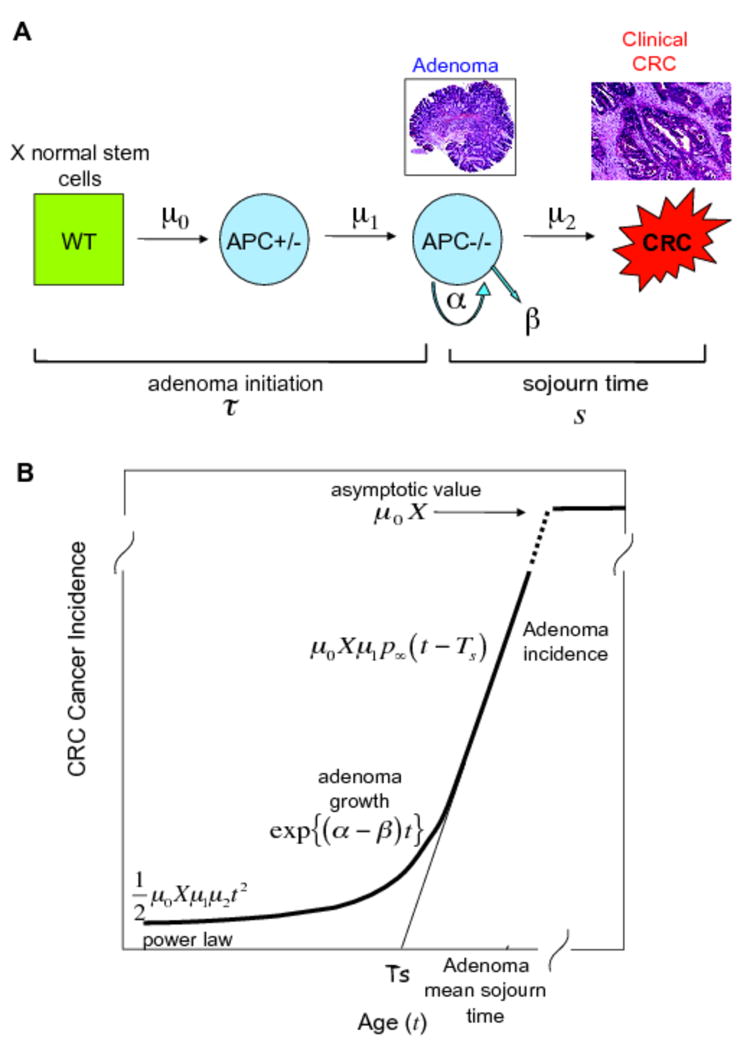
Our analyses were based on a class of multistage carcinogenesis models that allow for the sequential random accumulation of a specific number of mutations (or epigenetic events) prior to the initiation of a premalignant lesion (here the adenoma). We previously showed that the most parsimonious model, consistent with the overall incidence of CRC in SEER and with the known pathogenesis of microsatellite-stable CRC, is a 4-stage model(17, 19). This model posits two rare rate-limiting events and a high rate of asymmetric stem cell divisions (reflecting transient amplification in a crypt) for the initiation of an adenomatous polyp (adenoma) and is consistent with molecular evidence showing that adenomas frequently show biallelic inactivation of the Adenomatous Polyposis Coli (APC) gene(20, 21). Baseline exploratory analyses of CRC incidence data by subsite (proximal, distal, rectum) also revealed that a simpler 3-stage model, which requires only two rare rate-limiting events for the initiation of an adenoma, is equally consistent with the CRC incidence in SEER. This model also agrees with the notion that tumor initiation requires biallelic inactivation of a tumor suppressor locus such as, but not exclusively, the APC gene(17). The 3-stage model parameters are: the number of susceptible stem cells, X, the mutation rate of the first hit at the APC gene locus, μ0, the mutation rate of the second copy of the APC gene, μ1, the adenoma cell division rate α, the adenoma cell death or differentiation rate β, and the malignant transformation rate μ2 (Figure 1A). The mathematical treatment of these models and derivation of the corresponding hazard and survival functions are described elsewhere(17, 19).
Figure 1.
A. Three-stage CRC carcinogenesis model. The model agrees with the notion that adenoma initiation requires the biallelic inactivation of the APC gene. The parameters are: the number of susceptible stem cells, X, the mutation rate of the first hit at the APC gene, μ0, the mutation rate of the second copy of the APC gene, μ1, the adenoma cell division rate, α, the adenoma cell death or differentiation rate, β, and the malignant transformation rate, μ2.
B. The hazard (or incidence) function of the model (as a function of age t) exhibits three distinct phases that reveal the carcinogenic process in reverse. In particular, for older ages the hazard increases linearly reflecting the incidence of adenomas. The line approximating this (linear) phase has an intersect with the age axis that represents the mean time between adenoma initiation and its conversion into a clinical carcinoma (Ts). For mid-ages, the hazard rises exponentially, with a rate equal to the net growth rate of adenomas (α-β). For younger ages, the hazard increases as a power of age, consistent with the Armitage-Doll theory of multistage carcinogenesis
We have previously shown that the hazard (or incidence) function of these models (as a function of age t) exhibits three distinct phases that reveal the carcinogenic process in reverse(17). In particular, for older ages the hazard increases linearly reflecting the incidence of adenomas in the tissue (Figure 1B). The line approximating this (linear) phase has an intersect with the age axis that represents the mean sojourn time of an adenoma to become malignant (Ts), i.e., the mean time between the initiation of an adenoma and its conversion into a clinical carcinoma. For mid-ages, the hazard rises exponentially, with a rate equal to the net growth rate of adenomas (α-β). For younger ages, the hazard increases as a power of age. We have also shown previously that this behavior of the incidence function can be uniquely described by 3 parameter combinations (17), which are identified in the next section.
Reported outcomes
We estimated the following biological parameters within the models: the slope of the adenoma incidence (μ0Xμ1p∞), as a proxy of adenoma initiation, where p∞ denotes the adenoma survival probability (i.e., the asymptotic probability that a polyp does not become extinct); the growth rate of adenomas(α-β); the mean sojourn time of an adenoma (Ts); and the adenoma conversion rate(μ2/p∞).
Adjustments for secular trends
In the model, the effect of age is modeled parametrically; whereas secular trends (i.e. birth cohort and calendar-year effects) are modeled non-parametrically. The impact of screening on CRC incidence is captured indirectly by the calendar-year effects. For a given age t, birth cohort b and calendar year (period) c, the age-specific incidence is estimated by
| (1) |
where θc and θb are coefficients that modify the incidence function predicted by the multistage process (hmult(t)) allowing for non-specific calendar and birth cohort trends. Conforming with the format in which the SEER and UK data are distributed, we stratify the data in age groups (0–4 yrs, 5–9 yrs,…, 80–84 yrs, 85+ yrs) and into 34 calendar years (1973–2006). Age group 85+ was excluded for men due to rapidly declining person years after age 85. We then fitted 3- stage clonal expansion models to the number of observed proximal and distal colonic cancers and rectal cancers stratified by age group and calendar year. We obtain parameter estimates by maximizing the likelihood across all age-calendar strata assuming that the number of cases in each stratum is Poisson distributed with mean Icj(ti) PYij, where PYij denotes the number of person years in age group i and calendar year j. All analyses were done by sex, country and subsite. 95% confidence intervals (CIs) of all parameter estimates were computed using the Markov Chain Monte Carlo method.
Adenoma prevalence simulations
We used the methods developed by Jeon et al (18) to derive estimates of the prevalence of proximal, distal and rectum adenomas in asymptomatic individuals of ages 40, 45, 50, 55,…., 80 simulating 100,000 asymptomatic individuals for each age band and computing the fraction of these individuals with adenomas larger than 1 mm in size. The model expressed sizes in terms of the number of tumor (forming) stem cells and to transform these into diameter units, we assumed that a 1 mm adenoma contains about 500,000 cells(22). Of these cells, for the main model, we assumed that 1%(23) are tumorigenic, i.e., 5000 cells in a 1 mm adenoma.
Sensitivity analyses
We tested a number of critical modeling assumptions using sensitivity analyses. First, recognizing that the assumption of the molecular pathway of biallelic inactivation of a tumor suppressor gene may prevail in only 60% of CRCs, we repeated model analyses dropping histological types (namely mucinous adenocarcinomas: ICD-O-3 codes, 8480 and 8481) which appear to develop by molecular pathways that may not be captured adequately by our choice of model, i.e., pathways characterized by high levels of microsatellite instability (MSI-H) and/or a CpG island methylator phenotype (CIMP-high)(24). Second, to test if the patterns found in the biological parameter estimates were robust against the choice of period, we repeated analyses restricting the model fits to the periods; 1973–1984, 1985–1994, and 1995–2006, which broadly correspond in the US to periods of low screening, mainly flexible sigmoidoscopy screening, and mainly colonoscopy screening, respectively(8). Although we do not model the effect of screening and intervention (i.e. polypectomy) explicitly here, the impact of screening on CRC incidence is captured effectively by adjusting the common (i.e. unperturbed) incidence function for secular trends as described in equation (1). Our previous work(18) demonstrated how interventions can be modeled as a stochastic process within the multistage model framework. Third, for the UK data, where there was a high proportion of cancers defined as colon NOS, we repeated model fitting either excluding the NOS cases or recoded them in equal proportions for proximal and distal colon subsites. Finally, for the adenoma prevalence simulations, we repeated the model fitting assuming that 6.5% of cells in an adenoma are tumorigenic stem cells(25).
Results
Baseline characteristics
Table 1 shows the baseline characteristics for both registries. For the study period (1973 to 2006), there were 340,582 (men: 170,754; females: 169,828) cancers among Whites from the SEER database and 887,454 (men: 451,030; females: 436,424) cancers from the ONS database. Proportionally, cancers arising in the rectum were higher in the UK population, especially for men. For both registries the mean ages of cancer detections were older for right-sided cancers in both sexes.
Table 1.
Baseline characteristics
| Men | Women | Total | |
|---|---|---|---|
| US (SEER Whites 1973–2006) | |||
| No. of cancers (%) | |||
| Proximal colon | 59409 (34) | 74369 (44) | 133778 (39) |
| Distal colon | 55575 (33) | 51168 (30) | 106743 (32) |
| Rectum | 55770 (33) | 44291 (26) | 100061 (29) |
| Mean age (SD) (years) | |||
| Proximal colon | 70.23 (12.15) | 73.36 (11.76) | |
| Distal colon | 68.43 (11.53) | 69.75 (12.44) | |
| Rectum | 67.01 (11.89) | 69.17 (12.91) | |
| UK (ONS 1973–2006) | |||
| No. of cancers (%) | |||
| Proximal colon | 125198 (28) | 161719 (37) | 286917 (32) |
| Distal colon | 134652 (30) | 133590 (31) | 268242 (30) |
| Rectum | 191180 (42) | 141115 (32) | 332295 (38) |
| Mean age (SD) (years) | |||
| Proximal colon | 69.67 (11.59) | 72.80 (11.47) | |
| Distal colon | 69.31 (10.74) | 70.22 (11.68) | |
| Rectum | 68.61 (10.91) | 71.24 (11.81) | |
SD: standard deviation.
Model fit
We fitted 3-stage models to sex-specific CRC incidences among Whites in the SEER registry and separately for all cases in the UK registry. Each model was fitted independently for each anatomic subsite (supplemental material p5 to p10). We assumed identical calendar effects for distal colon and rectum, but independent calendar effects for proximal colon, and as screening practices have mainly changed with calendar year rather than birth cohort, we assumed identical birth cohort effects across all CRC subsites.
Calendar and birth-cohort effects for US and UK
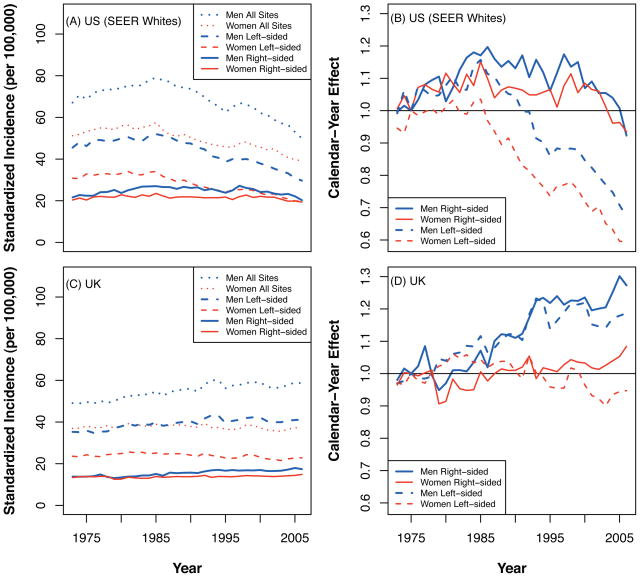
For the SEER data, the standardized incidences (per 100,000 populations) for White men were: 67.1 in 1973; peaking in 1985 at 79.0; and declining to 49.9 in 2006. For White women in the US, the respective incidences were: 51.2 in 1973, peaking in 1985 at 57.5; and declining to 39.1 in 2006 (Figure 2A). For the ONS data, the standardized incidences for men were: 49.1 in 1973, rising steadily to 58.6 in 2006; and for women were: 36.9 in 1973, remaining almost unchanged at 37.6 in 2006 (Figure 2C).
Figure 2.
Left. US and UK CRC incidence standardized to the US 2000 population by sex and subsite. Right. Estimated calendar-year effects.
The US calendar effects show a strong decreasing trend in the incidence of left-sided (distal colon and rectum) cancers since the mid 1980’s, but no such trend for right-sided (proximal colon) cancers for the age-calendar-cohort adjusted model (Figure 2B). No important sex differences were seen in the calendar effects. For the UK, no considerable differences in calendar effects were found between the subsites, although the model-based analysis suggested an increasing trend among men but not women (Figure 2D).
No clear birth-cohort trends are discernible in either dataset for births before 1945 (supplemental material p11). After 1945 (post WWII) there appears to be a slight (10–20%) increase in CRC risk in the US and a decrease of similar magnitude in the UK. However, the number of CRC cases for these recent birth cohorts is relatively small in both datasets and, with a limited number of post WWII cohorts, the significance of these nascent trends remains unclear.
Parameter estimates
The model parameter estimates by sex showed consistent patterns across subsites for US and UK data (Table 2). Specifically, the mean adenoma initiation rates (measured by the slope parameter) were higher for the proximal colon decreasing, in the main, through distal colon to rectum. The mean initiation rates for the distal colon and rectum, both in the US and UK, were significantly higher in men than in women. By contrast, the estimated mean adenoma growth (net cell proliferation) rates were lower in the proximal colon with a trend to higher values through the distal colon to the rectum, especially among men. As with mean initiation rates, the mean adenoma growth rates were generally higher in men compared with women.
Table 2.
Parameter estimates for CRC incidences for the US and UK
| Men |
Women |
|||||
|---|---|---|---|---|---|---|
| Proximal Colon | Distal Colon | Rectum | Proximal Colon | Distal Colon | Rectum | |
| US (SEER) | ||||||
| Adenoma initiation rate (μ0Xμ1P∞) | 10.42e-05 (9.39e-05, 10.82e-05) | 6.37e-05 (6.00e-05, 6.57e-05) | 4.69e-05 (4.42e-05, 4.82e-05) | 10.10e-05 (9.52e-05, 10.60e-05) | 3.79e-05 (3.60e-05, 3.92e-05) | 2.74e-05 (2.62e-05, 2.85e-05) |
| Adenoma growth rate (α – β) | 0.1438 (0.1415, 0.1463) | 0.1862 (0.1821, 0.1898) | 0.1985 (0.1941, 0.2032) | 0.1374 (0.1351, 0.1393) | 0.1769 (0.1729, 0.1816) | 0.1774 (0.1714, 0.1823) |
| Mean sojourn time* (Ts) | 63.7 (63.1, 64.3) | 54.2 (53.8, 54.6) | 50.1 (49.7, 50.4) | 65.0 (64.5, 65.5) | 50.6 (50.1, 51.0) | 48.3 (47.9, 48.9) |
| Adenoma conversion rate μ2/P∞ | 1.52e-05 (1.41e-05, 1.63e-05) | 0.77e-05 (0.68e-05, 0.88e-05) | 0.95e-05 (0.82e-05, 1.11e-05) | 1.82e-05 (1.71e-05, 1.96e-05) | 2.28e-05 (1.97e-05, 2.62e-05) | 3.34e-05 (2.90e-05, 3.99e-05) |
| UK (ONS) | ||||||
| Adenoma initiation rate (μ0Xμ1P∞) | 7.34e-05 (6.83e-05, 7.62e-05) | 5.38e-05 (5.16e-05, 5.52e-05) | 5.44e-05 (5.26e-05, 5.54e-05) | 6.53e-05 (6.13e-05, 6.65e-05) | 2.74e-05 (2.63e-05, 2.78e-05) | 2.98e-05 (2.85e-05, 3.02e-05) |
| Adenoma growth rate (α – β) | 0.1218 (0.1200, 0.1235) | 0.1502 (0.1479, 0.1527) | 0.1714 (0.1688, 0.1738) | 0.1200 (0.1186, 0.1219) | 0.1536 (0.1509, 0.1568) | 0.1477 (0.1454, 0.1507) |
| Mean sojourn time* (Ts) | 66.6 (65.9, 67.3) | 59.3 (58.8, 59.7) | 53.7 (53.4, 54.0) | 66.0 (65.4, 66.5) | 53.3 (52.9, 53.7) | 54.0 (53.5, 54.3) |
| Adenoma conversion rate μ2/P∞ | 3.66e-05 (3.49e-05, 3.83e-05) | 2.03e-05 (1.89e-05, 2.18e-05) | 1.73e-05 (1.61e-05, 1.88e-05) | 4.34e-05 (4.13e-05, 4.56e-05) | 4.26e-05 (3.85e-05, 4.63e-05) | 5.09e-05 (4.65e-05, 5.45e-05) |
Mean sojourn time: time in years between the appearance of the first cell in an adenoma to the clinical detection of a carcinoma.
Unless otherwise stated, values in parenthesis are 95% confidence intervals.
The adenoma mean sojourn times (the time it takes from an adenoma’s origin to its transition into a clinical carcinoma) were longer for proximal colon compared with the distal colon to rectum. These patterns were similar for the US and UK, although the mean sojourn times estimated for the UK lagged behind those for the US by 2 to 5 years.
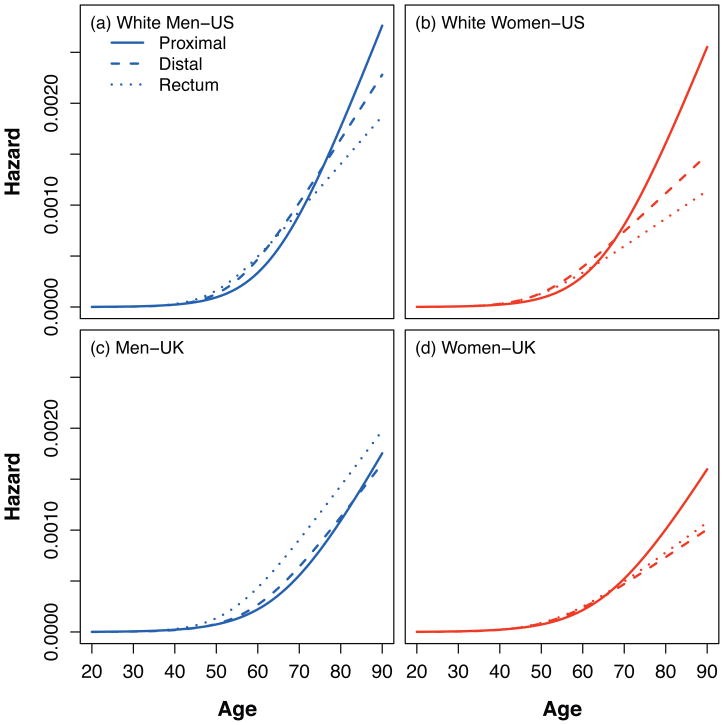
Hazard functions for US and UK
The estimated hazard functions represent the combined effects of differential tumor initiation rates and tumor growth rates (adjusted for calendar-year and birth-cohort effects) on CRC incidences and are shown for the US and UK in Figure 3. For ages less than 70 years, the pattern was one in which left-sided cancers were more prevalent; after 70 years, the pattern was one in which right-sided cancers were more dominant. This pattern prevailed for both sexes in the US and for women in the UK.
Figure 3.
Three-stage CRC model hazard by country, sex and subsite. The figure shows the combined effects of differential tumor initiation rates and tumor growth rates on the estimated hazard functions that represent the intrinsic age-effects on CRC risk. In all panels, left-sided lesions dominate during early ages and are more prevalent, whereby in older ages, there is a right-sided dominance.
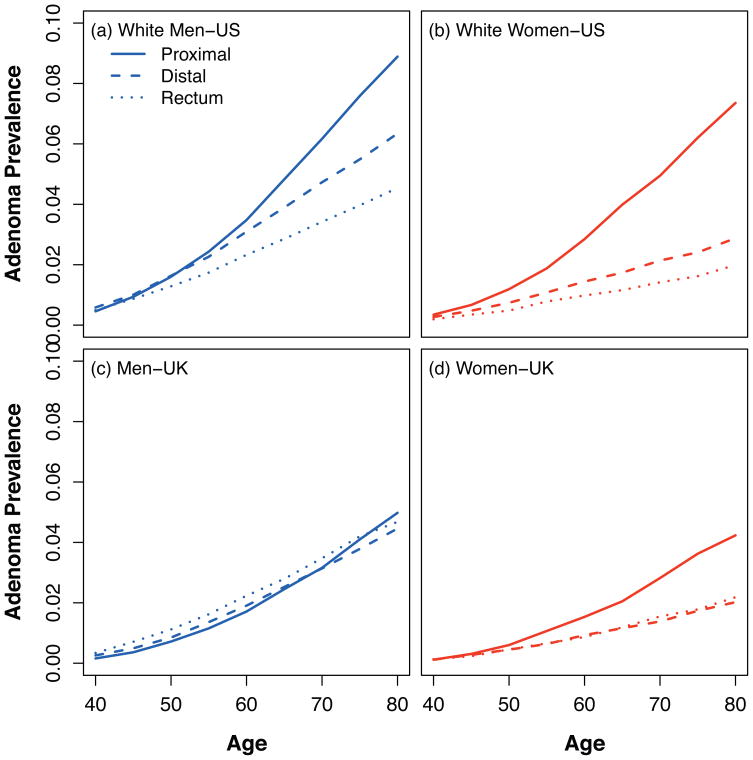
Simulated adenoma prevalence
The predicted prevalence of adenomas of at least 1 mm for men and women in the US and UK are shown in Figure 4. The relative prevalence of adenomas increased with age, was higher in men compared with women in both registries, and was higher for proximal colon versus distal colon and rectum for both sexes in the US and women in the UK. In contrast to the cancer incidence data, there was no ‘cross-over’ at the age of 70 years, with the exception of men in the UK.
Figure 4.
Predicted adenoma (≥1mm) prevalence in asymptomatic individuals by country, sex and subsite. We assume that there are about 500,000 cells in a 1mm adenoma (Pinsky, JTB 2000) and that about 1% of those are stem cells (Boman, JCO 2008). The relative prevalence of right-sided versus left-sided adenomas increases with age in all panels.
Sensitivity analyses
Histological subtypes were recorded in the SEER database, but not the ONS. There were 32,504 (men, 15,072: women, 17,432) individuals or 9.5% with a histological diagnosis of mucinous adenocarcinoma - in turn, this histological subtype was most prevalent in the proximal colon. When these were excluded and the models repeated, there were no material changes in the patterns of parameter estimates (supplemental material p12). We also recalculated the parameter estimates for the US and UK data separately for the periods 1973–1984, 1985–1994, and 1995–2006, and found similar patterns as the main analysis (supplemental material p13-p15). We repeated the model fitting of the UK data re-allocating colon NOS cases either by exclusion or in equal amounts to proximal and distal colon subsites, and found consistent results (supplemental material p16-p17). Finally, we repeated the adenoma prevalence simulations assuming that 6.5%, rather than 1%, of cells are tumorigenic stem cells(25), and obtained similar results as with the baseline assumption (supplemental material p18).
Discussion
Main findings
This study derived estimates of tumor initiation rates and tumor growth rates for right-and left-sided CRCs using data on more than one million cancers from two nationally representative populations and previously developed mathematical models for CRC development. Despite considerable differences in background screening practices between the two countries, the patterns of parameter estimates were very similar; namely, tumor initiation rates were highest in the right-sided, whereas tumor growth rates were highest for left-sided tumors. In almost all scenarios, these rates are greater in men than women. The net effect of these two fundamental processes in carcinogenesis (expressed by the hazard function) is consistent with a model in which the incidences of left-sided CRCs dominate over right-sided CRCs before the age of 70 years, with a reversal in the respective incidences thereafter, independent of screening prevalence. A notable unexplained exception occurred for men in the UK, who are at a higher risk for developing left-sided cancers throughout life.
Study limitations and strengths
The study has a number of limitations. First, in common with any mathematical model, our modeling framework is a simplification of the biological complexity of carcinogenesis and neglects the influence of various endogenous and exogenous modifiers of CRC risk – for example, obesity(26), physical inactivity(27), alcohol(28), smoking(29), and decreased risk from hormonal replacement therapy in women(30) – covariate information that was not available for this study. Second, implicit in the 3-stage clonal expansion model is the assumption of two rate-limiting events representing the biallelic inactivation of a tumor suppressor gene (APC gene and/or others) for tumor initiation, which account for 60% to 80% of sporadic CRC cases(24). Tumors that exhibit MSI-H and/or CIMP-high may require more than two rate-limiting events for the initiation of neoplastic growth. We addressed this by excluding mucinous adenocarcinomas, where MSI-H and/or CIMP-high are prevalent, and found no material differences in the parameter estimates. Third, the high proportion of colon NOS cancers registered in the UK database may lead to misclassification biases. To address this, we randomly recoded the colon NOS cases proportionate to the subsite distributions at the corresponding age and year of diagnosis, and performed additional sensitivity analyses, and found that these did not alter our conclusions. Fourth, while our integrated biological approach of modeling incidence implicitly predicts the age-specific adenoma prevalence(18), these estimates cannot be compared directly with empirical data on observed adenoma prevalence. The model expresses sizes in terms of the number of tumor forming stem cells, which currently cannot be identified due to a lack of appropriate markers. We dealt with these uncertainties using estimates tumor stem cell percentages of 1%(23) and 6.5%(25), the latter estimate consistent with that inferred from DNA methylation patterns in CRC assuming 50% asymmetric divisions(31). Our model predicts lower age-specific adenoma prevalence rates than are typically observed in colonoscopy studies of asymptomatic subjects(32–36) – 12.5% for age 40 to 49 years; 19.1% to 28.0% for 50 to 59 years; 32.0% to 44.7% for 60 to 69 years; and 33.3% to 57.9% for 70+ years - although the cited series were either exclusively from male subjects(33, 34) or predominantly from male subjects(32, 35, 36). However, our model did correctly predict a greater adenoma prevalence in men than women(37, 38) and did predict differential changes with increasing age(38).
The use of a biologically-based multistage model for CRC has several strengths. First, the mathematical model has previously been developed(17, 19, 39) and validated in clinical scenarios, including the evaluation of CRC screening strategies(18). Additionally, the model has been used to explore the potential benefits and risks for CRC resulting from folic acid supplementation(40) and used to describe the age-specific incidence of pancreatic cancer (17). Second, having previously validated this model against US CRC incidence data(17, 19, 39), we extended this validation to UK CRC incidence data and arrived at similar patterns for the parameter estimates suggesting common physiological gradients in colon and rectum for these two populations. Third, this study used two large established registries with three decades of data and from comparable westernized populations allowing us to focus on the subsite-specific biological parameters that determine tumor development. Despite differences in screening practices between the two countries, after adjustment for calendar-year effects to capture the impact of screening, we obtained consistent estimates for the biological parameters (by subsite) for the two populations suggesting that the differential age- and sex-related adenoma prevalences and CRC incidences between proximal and distal colon and rectum are real and not caused by screening.
Comparison with other literature
In common with previous epidemiological studies(11–13), we have shown that, in the US, there has been a decline in CRC incidences since the mid-1980s and that this decline is limited to left-sided cancers.
The derived model parameters suggest higher adenoma initiation rates in the proximal colon compared with distal colon and rectum, gradients which might arise from site-specific differences in the number of susceptible tissue stem cells. In turn, the numbers of intestinal crypts per individual are related to colon lengths and surface areas. Consistent with the relative estimates of the adenoma initiation rates in our study, using measurements from barium enema radiographs, Sadahiro and colleagues(41, 42) reported larger surface areas in the proximal colon relative to the distal colon, although their estimated proximal colon surface area was 1.6 times that of the distal colon in women, while our estimate of the adenoma initiation rate in the proximal colon in women was 2.6 times that of the distal colon. In general, adenoma initiation rates are higher in men than in women, especially in the distal colon and rectum. This finding is consistent with measurements from magnetic imaging colonoscopy that show greater left-sided colonic lengths in men compared with women(43). The lower initiation rates in the rectum are consistent with cell proliferation studies demonstrating significantly lower labeling indices in the rectum(44), although other studies have reported no differences in the mutational load between proximal and distal colon(45). Tumor growth rates of premalignant lesions increase from right to left colon, a finding that is consistent with a similar gradient noted for labeling index measurements of cell proliferation in chemically-induced rat colon tumors(46).
The adenoma mean sojourn times (time between the appearance of the first cell in an adenoma to the clinical detection of a carcinoma) that we report (>50 years) are estimations, not assumptions, obtained independently for the US and UK data. These durations may seem long, but are not inconsistent with estimations from lesion sequencing studies (47). These should be contrasted with the mean dwell time of observable adenomas progressing toward clinical cancer and the pre-symptomatic duration of CRC (about 20 years and 6.7 years, respectively, as estimated by Landsdorp-Vogelaar and colleagues (48)). However, these estimates are for colon and rectal tumors combined, thus do not differentiate right versus left colon. Our estimates of the adenoma sojourn time differ between right (slower) and left (faster) colon by about 10 to 15 years.
Clinical implications and future research
Our findings support the hypothesis that there is a differential preventive effect on right-and left-sided cancers from screening endoscopy. They lend support to the study by Baxter and colleagues(14) who found that colonoscopy reduces significantly left-sided CRC mortality (by 70%), but does not appear to have a significant effect on right-sided CRC mortality. While external factors such as missed proximal colonic lesions and/or incomplete coverage of the whole colon during colonoscopy may be relevant, this study argues that differences in the biological characteristics of right- and left-sided tumors are equally relevant. It is often cited that right-sided adenomas grow faster than left-sided, implying that there might be a smaller window of opportunity to detect polyps in the proximal colon by screening – but this is mainly based on MSI-H tumors associated with hereditary non-polyposis CRC(49). In fact, our analyses show the opposite: proximal colon polyps grow slower than polyps in distal colon and rectum in a sex-specific manner, which is consistent with the delayed onset of right-sided CRC relative to left-sided. Whether or not right-sided malignancies grow faster (and therefore escape detection) requires further study and preferably a model-based analysis that includes additional data on screen detected colorectal neoplasia. For general population CRC screening, our findings point to the importance of designing screening schedules that recognize the differences between women and men(37, 38, 50); and to the potential benefits from targeting screening strategies differentially to the right- and left-sided lesions at different ages.
Our study provides additional rationale to reconsider screening paradigms of colonoscopy versus FOB versus flexible sigmoidoscopy. From a pragmatic point of view, the CRC model described here may assist the design of future clinical studies that integrate optimal modalities and target strategies. For example, in an aging population with limited resources, flexible sigmoidoscopy may be more cost effective under ages of 70 years, but thereafter screening colonoscopy may be more appropriate, assuming it has adequate efficacy in the proximal colon.
In view of these findings future colonoscopy trials should appropriately power recruitment to allow a priori stratified analyses for right- and left-sided neoplastic endpoints. Moreover, there is a need for translational research to better characterize the impact of risk factors such as obesity and smoking on relevant biological endpoints (e.g. cell proliferation, differentiation and apoptosis) during adenoma initiation and progression. Finally, there is an opportunity to “piggy-back” the CRC risk models used in the cancer control field onto cancer stem cell research recognizing the recent identification of key tumor stem cell markers in CRC – for example, Lgr5(25) and CD133(51). Promising are studies of DNA-methylation patterns to “fingerprint” the age of adenomas(31) which is a key parameter of virtually all CRC models in the field. Such improvements in modeling will undoubtedly lead to more reliable risk predictions and thereby better inform policy-makers on cost-effective screening and intervention strategies to prevent CRC more effectively at the population level.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Health Grants RO1 CA107028 and RO1 CA119224. AGR is supported by a HEFCE “new blood” senior lectureship. RM acknowledges the support of the Division of Mathematical Modeling at the UBC Centre for Disease Control.
Footnotes
Potential conflicts of interest
There are no conflicts of interest.
References
- 1.American Cancer Society. [accessed 4th March 2010];Colorectal Cancer Facts & Figures. 2008–2010 http://www.cancer.org/downloads/STT/F861708_finalforweb.pdf.
- 2. [accessed 4th March 2010];Cancer Research UK Cancer Statistics. http://info.cancerresearchuk.org/cancerstats/incidence/index.htm.
- 3.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. minnesota colon cancer control study. N Engl J Med. 1993;328(19):1365–71. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 4.Modeling to guide public health research and priorities. [accessed 4th March 2010];Cancer Intervention and Surveillance Modeling Network. http://cisnet.cancer.gov/colorectal/
- 5.Muller AD, Sonnenberg A. Protection by endoscopy against death from colorectal cancer. A case-control study among veterans. Arch Intern Med. 1995;155(16):1741–8. doi: 10.1001/archinte.1995.00430160065007. [DOI] [PubMed] [Google Scholar]
- 6.Kahi CJ, Imperiale TF, Juliar BE, Rex DK. Effect of screening colonoscopy on colorectal cancer incidence and mortality. Clin Gastroenterol Hepatol. 2009;7(7):770,5. doi: 10.1016/j.cgh.2008.12.030. quiz 711. [DOI] [PubMed] [Google Scholar]
- 7.Brenner H, Hoffmeister M, Brenner G, Altenhofen L, Haug U. Expected reduction of colorectal cancer incidence within 8 years after introduction of the german screening colonoscopy programme: Estimates based on 1,875,708 screening colonoscopies. Eur J Cancer. 2009;45(11):2027–33. doi: 10.1016/j.ejca.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 8.Gross CP, Andersen MS, Krumholz HM, McAvay GJ, Proctor D, Tinetti ME. Relation between medicare screening reimbursement and stage at diagnosis for older patients with colon cancer. JAMA. 2006;296(23):2815–22. doi: 10.1001/jama.296.23.2815. [DOI] [PubMed] [Google Scholar]
- 9.Harewood GC, Lieberman DA. Colonoscopy practice patterns since introduction of medicare coverage for average-risk screening. Clin Gastroenterol Hepatol. 2004;2(1):72–7. doi: 10.1016/s1542-3565(03)00294-5. [DOI] [PubMed] [Google Scholar]
- 10.NHS. [accessed 4th March 2010];Bowel Cancer Screening Programme. http://www.cancerscreening.nhs.uk/bowel/
- 11.Rabeneck L, Davila JA, El-Serag HB. Is there a true shift to the right colon in the incidence of colorectal cancer? Am J Gastroenterol. 2003;98:1400–9. doi: 10.1111/j.1572-0241.2003.07453.x. [DOI] [PubMed] [Google Scholar]
- 12.Wu X, Chen VW, Martin J, et al. Subsite-specific colorectal cancer incidence rates and stage distributions among asians and pacific islanders in the united states, 1995 to 1999. Cancer Epidemiol Biomarkers Prev. 2004;13:1215–22. [PubMed] [Google Scholar]
- 13.Cress RD, Morris C, Ellison GL, Goodman MT. Secular changes in colorectal cancer incidence by subsite, stage at diagnosis, and race/ethnicity, 1992–2001. Cancer. 2006;107(5 Suppl):1142–52. doi: 10.1002/cncr.22011. [DOI] [PubMed] [Google Scholar]
- 14.Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer: A population-based, case- control study. Ann Intern Med. 2009:150. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 15.Bressler B, Paszat LF, Chen Z, Rothwell DM, Vinden C, Rabeneck L. Rates of new or missed colorectal cancers after colonoscopy and their risk factors: A population-based analysis. Gastroenterology. 2007;132(1):96–102. doi: 10.1053/j.gastro.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 16.Ransohoff DF. How much does colonoscopy reduce colon cancer mortality? Ann Intern Med. 2009:150. doi: 10.7326/0003-4819-150-1-200901060-00308. [DOI] [PubMed] [Google Scholar]
- 17.Meza R, Jeon J, Moolgavkar SH, Luebeck EG. Age-specific incidence of cancer: Phases, transitions, and biological implications. Proc Natl Acad Sci U S A. 2008;105:16284–9. doi: 10.1073/pnas.0801151105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeon J, Meza R, Moolgavkar SH, Luebeck EG. Evaluation of screening strategies for pre-malignant lesions using a biomathematical approach. Math Biosci. 2008;213:56–70. doi: 10.1016/j.mbs.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luebeck EG, Moolgavkar SH. Multistage carcinogenesis and the incidence of colorectal cancer. Proc Natl Acad Sci U S A. 2002;99(23):15095–15100. doi: 10.1073/pnas.222118199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bodmer WF, Bailey CJ, Bodmer J, et al. Localization of the gene for familial adenomatous polyposis on chromosome 5. Nature. 1987;328(6131):614–6. doi: 10.1038/328614a0. [DOI] [PubMed] [Google Scholar]
- 21.Kinzler KW, Nilbert MC, Su LK, et al. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253(5020):661–5. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 22.Pinsky PF. A multi-stage model of adenoma development. J Theor Biol. 2000;207:129–43. doi: 10.1006/jtbi.2000.2148. [DOI] [PubMed] [Google Scholar]
- 23.Boman BM, Huang E. Human colon cancer stem cells: A new paradigm in gastrointestinal oncology. J Clin Oncol. 2008;26:2828–38. doi: 10.1200/JCO.2008.17.6941. [DOI] [PubMed] [Google Scholar]
- 24.Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50(1):113–30. doi: 10.1111/j.1365-2559.2006.02549.x. [DOI] [PubMed] [Google Scholar]
- 25.Barker N, Ridgway RA, van Es JH, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–11. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 26.Harriss DJ, Atkinson G, George K, et al. Lifestyle factors and colorectal cancer risk (1): Systematic review and meta-analysis of associations with body mass index. Colorectal Dis. 2009;11(6):547–63. doi: 10.1111/j.1463-1318.2009.01766.x. [DOI] [PubMed] [Google Scholar]
- 27.Harriss DJ, Atkinson G, Batterham A, et al. Lifestyle factors and colorectal cancer risk (2): A systematic review and meta-analysis of associations with leisure-time physical activity. Colorectal Dis. 2009;11(7):689–701. doi: 10.1111/j.1463-1318.2009.01767.x. [DOI] [PubMed] [Google Scholar]
- 28.Moskal A, Norat T, Ferrari P, Riboli E. Alcohol intake and colorectal cancer risk: A dose-response meta-analysis of published cohort studies. Int J Cancer. 2007;120(3):664–71. doi: 10.1002/ijc.22299. [DOI] [PubMed] [Google Scholar]
- 29.Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and colorectal cancer: A meta-analysis. JAMA. 2008;300(23):2765–78. doi: 10.1001/jama.2008.839. [DOI] [PubMed] [Google Scholar]
- 30.Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med. 2004;350(10):991–1004. doi: 10.1056/NEJMoa032071. [DOI] [PubMed] [Google Scholar]
- 31.Siegmund KD, Marjoram P, Woo YJ, Tavare S, Shibata D. Inferring clonal expansion and cancer stem cell dynamics from DNA methylation patterns in colorectal cancers. Proc Natl Acad Sci U S A. 2009;106(12):4828–33. doi: 10.1073/pnas.0810276106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson DA, Gurney MS, Volpe RJ, et al. A prospective study of the prevalence of colonic neoplasms in asymptomatic patients with an age-related risk. Am J Gastroenterol. 1990;85(8):969–74. [PubMed] [Google Scholar]
- 33.DiSario JA, Foutch PG, Mai HD, Pardy K, Manne RK. Prevalence and malignant potential of colorectal polyps in asymptomatic, average-risk men. Am J Gastroenterol. 1991;86:941–5. [PubMed] [Google Scholar]
- 34.Lieberman DA, Smith FW. Screening for colon malignancy with colonoscopy. Am J Gastroenterol. 1991;86(8):946–51. [PubMed] [Google Scholar]
- 35.Rex DK, Lehman GA, Ulbright TM, et al. Colonic neoplasia in asymptomatic persons with negative fecal occult blood tests: Influence of age, gender, and family history. Am J Gastroenterol. 1993;88(6):825–31. [PubMed] [Google Scholar]
- 36.Imperiale TF, Wagner DR, Lin CY, Larkin GN, Rogge JD, Ransohoff DF. Results of screening colonoscopy among persons 40 to 49 years of age. N Engl J Med. 2002;346:1781–5. doi: 10.1056/NEJM200206063462304. [DOI] [PubMed] [Google Scholar]
- 37.Regula J, Rupinski M, Kraszewska E, et al. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 2006;355(18):1863–72. doi: 10.1056/NEJMoa054967. [DOI] [PubMed] [Google Scholar]
- 38.Lieberman DA, Holub JL, Moravec MD, Eisen GM, Peters D, Morris CD. Prevalence of colon polyps detected by colonoscopy screening in asymptomatic black and white patients. JAMA. 2008;300:1417–22. doi: 10.1001/jama.300.12.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meza R, Luebeck EG, Moolgavkar SH. Gestational mutations and carcinogenesis. Math Biosci. 2005;197(2):188–210. doi: 10.1016/j.mbs.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 40.Luebeck EG, Moolgavkar SH, Liu AY, Boynton A, Ulrich CM. Does folic acid supplementation prevent or promote colorectal cance r? results from model-based predictions. Cancer Epidemiol Biomarkers Prev. 2008;17:1360–7. doi: 10.1158/1055-9965.EPI-07-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sadahiro S, Ohmura T, Saito T, Suzuki S. Relationship between length and surface area of each segment of the large intestine and the incidence of colorectal cancer. Cancer. 1991;68:84–7. doi: 10.1002/1097-0142(19910701)68:1<84::aid-cncr2820680117>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 42.Sadahiro S, Ohmura T, Yamada Y, Saito T, Taki Y. Analysis of length and surface area of each segment of the large intestine according to age, sex and physique. Surg Radiol Anat. 1992;14:251–7. doi: 10.1007/BF01794949. [DOI] [PubMed] [Google Scholar]
- 43.Shah SG, Saunders BP, Brooker JC, Williams CB. Magnetic imaging of colonoscopy: An audit of looping, accuracy and ancillary maneuvers. Gastrointest Endosc. 2000;52(1):1–8. doi: 10.1067/mge.2000.107296. [DOI] [PubMed] [Google Scholar]
- 44.Potten CS, Kellett M, Roberts SA, Rew DA, Wilson GD. Measurement of in vivo proliferation in human colorectal mucosa using bromodeoxyuridine. Gut. 1992;33:71–8. doi: 10.1136/gut.33.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101:403–8. doi: 10.1002/ijc.10635. [DOI] [PubMed] [Google Scholar]
- 46.Ma QY, Williamson KE, Rowlands BJ. Variability of cell proliferation in the proximal and distal colon of normal rats and rats with dimethylhydrazine induced carcinogenesis. World J Gastroenterol. 2002;8:847–52. doi: 10.3748/wjg.v8.i5.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones S, Chen WD, Parmigiani G, et al. Comparative lesion sequencing provides insights into tumor evolution. Proc Natl Acad Sci U S A. 2008;105:4283–8. doi: 10.1073/pnas.0712345105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lansdorp-Vogelaar I, van Ballegooijen M, Boer R, Zauber A, Habbema JD. A novel hypothesis on the sensitivity of the fecal occult blood test: Results of a joint analysis of 3 randomized controlled trials. Cancer. 2009;115(11):2410–9. doi: 10.1002/cncr.24256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jarvinen HJ, Aarnio M, Mustonen H, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118(5):829–34. doi: 10.1016/s0016-5085(00)70168-5. [DOI] [PubMed] [Google Scholar]
- 50.Brenner H, Hoffmeister M, Haug U. Should colorectal cancer screening start at the same age in european countries? contributions from descriptive epidemiology. Br J Cancer. 2008;99(3):532–5. doi: 10.1038/sj.bjc.6604488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445(7123):106–10. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.