Abstract
The title complex, [Fe(C5H5)(C13H11N2O3)], was prepared via self-assembly using ferrocenyl hydrazide and ethyl salicylate. The compound is potentially a tridentate ferrocene-based ligand. The conformation of the molecule allows the formation of an intramolecular N—H⋯O hydrogen bond involving the hydroxyl group. The CONHNHCO unit and the rings bonded to it are nearly coplanar. The crystal structure is stabilized by intermolecular O—H⋯O(carbonyl) and N—H⋯O(carbonyl) hydrogen bonds.
Related literature
For related literature about applications of ferrocene complexes, see: Beer (1992 ▶); Beer & Smith (1997 ▶); Long (1995 ▶); Miller & Epstein (1994 ▶); Nguyen et al. (1999 ▶).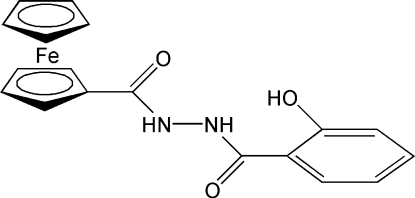
Experimental
Crystal data
[Fe(C5H5)(C13H11N2O3)]
M r = 364.18
Monoclinic,
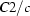
a = 20.680 (3) Å
b = 9.9673 (15) Å
c = 16.941 (3) Å
β = 121.704 (3)°
V = 2970.8 (8) Å3
Z = 8
Mo Kα radiation
μ = 1.03 mm−1
T = 293 (2) K
0.20 × 0.18 × 0.16 mm
Data collection
Bruker SMART APEX CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2000 ▶) T min = 0.820, T max = 0.852
7479 measured reflections
2611 independent reflections
1053 reflections with I > 2σ(I)
R int = 0.129
Refinement
R[F 2 > 2σ(F 2)] = 0.049
wR(F 2) = 0.086
S = 0.57
2611 reflections
230 parameters
3 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.48 e Å−3
Δρmin = −0.34 e Å−3
Data collection: SMART (Bruker, 2000 ▶); cell refinement: SAINT-Plus (Bruker, 2000 ▶); data reduction: SAINT-Plus; program(s) used to solve structure: SHELXS97 (Sheldrick, 1997a ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 1997a ▶); molecular graphics: SHELXTL (Sheldrick, 1997b ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536807065634/bh2153sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536807065634/bh2153Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N2—H2B⋯O3 | 0.860 (10) | 1.95 (2) | 2.631 (4) | 135 (3) |
| O3—H3B⋯O1i | 0.822 (10) | 1.908 (15) | 2.705 (4) | 163 (4) |
| N1—H1B⋯O2ii | 0.871 (10) | 2.03 (2) | 2.810 (4) | 148 (4) |
Symmetry codes: (i) 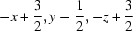 ; (ii)
; (ii) 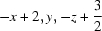 .
.
Acknowledgments
This work was financially supported by the Foundation of the Educational Department of Henan Province (No. 2007150012).
supplementary crystallographic information
Comment
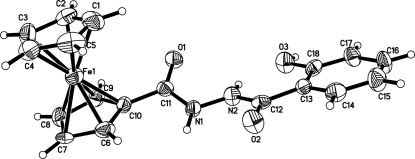
The synthesis, isolation and characterization of ferrocene in 1951 marked an important milestone in the evolution of modern organometallic chemistry. Potential applications in material sciences, such as molecular sensors (Beer, 1992; Beer & Smith, 1997), molecular magnetic materials (Miller & Epstein, 1994), and nonlinear optical materials (Nguyen et al., 1999; Long, 1995) attracted much attention. We report here the crystal structure of the title compound, (I), a new ferrocene-based complex (Fig. 1).
The title compound belongs to space group C2/c. The bond lengths O1?C11 and O2?C12 are 1.240 (5) and 1.233 (4) Å, respectively, as excepted for double bonds. The bond length O3—C18, 1.349 (5) Å, corresponds to a single bond. The N1—C11 and N2—C12 bond distances are 1.340 (5) and 1.343 (5) Å, respectively, which make clear they are in the normal range for N—C single bonds. The bond length N1—N2 = 1.381 (4) Å is also consistent with a single N—N bond. An intramolecular N2—H2B···O3 hydrogen bond is observed in the molecular structure.
In the crystal, molecules are connected by intermolecular hydrogen bonds involving carbonyl O atoms O2 and O3 as acceptor and N—H or O—H groups as donors.
Experimental
All reagents were commercially available and of analytical grade. Ferrocenyl hydrazide (1.22 g, 5 mmol) and ethyl salicylate (0.83 g, 5 mmol) were mixed in ethanol and refluxed for 7 h. The resulting red solid was filtered, washed with ethanol and dried under reduced pressure. Anal. Calc. for C18H16FeN2O3: C 59.37, H 4.43, N 7.69%. Found: C 59.48, H 4.31, N 7.52%.
Refinement
H atoms bonded to C atoms were positioned geometrically and refined as riding on their carrier atoms, with C—H bond lengths fixed to 0.93 (benzene ring) or 0.98 Å (Cp rings), and Uiso(H) = 1.2Ueq(carrier C). H atoms bonded to heteroatoms N1, N2 and O3 were located in a difference map and were freely refined as isotropic atoms, with restricted bond lengths: N—H = 0.87 (1) Å and O—H = 0.82 (1) Å.
Figures
Fig. 1.
The molecular structure of (I) with displacement ellipsoids drawn at the 50% probability level.
Crystal data
| [Fe(C5H5)(C13H11N2O3)] | F000 = 1504 |
| Mr = 364.18 | Dx = 1.628 Mg m−3 |
| Monoclinic, C2/c | Mo Kα radiation λ = 0.71073 Å |
| Hall symbol: -C 2yc | Cell parameters from 397 reflections |
| a = 20.680 (3) Å | θ = 2.3–28.0º |
| b = 9.9673 (15) Å | µ = 1.04 mm−1 |
| c = 16.941 (3) Å | T = 293 (2) K |
| β = 121.704 (3)º | Block, red |
| V = 2970.8 (8) Å3 | 0.20 × 0.18 × 0.16 mm |
| Z = 8 |
Data collection
| Bruker SMART APEX CCD area-detector diffractometer | 2611 independent reflections |
| Radiation source: fine-focus sealed tube | 1053 reflections with I > 2σ(I) |
| Monochromator: graphite | Rint = 0.129 |
| T = 293(2) K | θmax = 25.0º |
| 0.3° wide ω scans | θmin = 2.3º |
| Absorption correction: multi-scan(SADABS; Bruker, 2000) | h = −24→18 |
| Tmin = 0.820, Tmax = 0.852 | k = −11→11 |
| 7479 measured reflections | l = −20→20 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.049 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.086 | w = 1/[σ2(Fo2) + (0.02P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 0.57 | (Δ/σ)max < 0.001 |
| 2611 reflections | Δρmax = 0.48 e Å−3 |
| 230 parameters | Δρmin = −0.34 e Å−3 |
| 3 restraints | Extinction correction: none |
| Primary atom site location: structure-invariant direct methods |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Fe1 | 0.94540 (4) | 0.77786 (6) | 0.86429 (4) | 0.03507 (19) | |
| O1 | 0.83150 (16) | 0.4726 (3) | 0.79890 (19) | 0.0410 (9) | |
| O2 | 0.92978 (15) | 0.2865 (3) | 0.63670 (17) | 0.0437 (8) | |
| O3 | 0.75985 (16) | 0.1341 (3) | 0.6733 (2) | 0.0417 (8) | |
| H3B | 0.7289 (10) | 0.099 (3) | 0.683 (2) | 0.060 (9)* | |
| N1 | 0.9235 (2) | 0.3971 (3) | 0.7765 (2) | 0.0308 (10) | |
| H1B | 0.9700 (8) | 0.387 (4) | 0.790 (2) | 0.058 (16)* | |
| N2 | 0.87479 (18) | 0.2969 (4) | 0.7205 (2) | 0.0340 (9) | |
| H2B | 0.8365 (9) | 0.276 (3) | 0.7248 (17) | 0.034 (5)* | |
| C1 | 0.8498 (3) | 0.8152 (5) | 0.7406 (3) | 0.0489 (14) | |
| H1A | 0.8151 | 0.7473 | 0.6970 | 0.059* | |
| C2 | 0.8456 (3) | 0.8730 (4) | 0.8133 (3) | 0.0432 (13) | |
| H2A | 0.8071 | 0.8530 | 0.8287 | 0.052* | |
| C3 | 0.9052 (3) | 0.9656 (4) | 0.8594 (3) | 0.0451 (14) | |
| H3A | 0.9161 | 1.0216 | 0.9126 | 0.054* | |
| C4 | 0.9463 (3) | 0.9629 (4) | 0.8144 (3) | 0.0454 (14) | |
| H4A | 0.9919 | 1.0160 | 0.8322 | 0.055* | |
| C5 | 0.9131 (3) | 0.8688 (5) | 0.7419 (3) | 0.0529 (15) | |
| H5A | 0.9302 | 0.8462 | 0.6995 | 0.064* | |
| C6 | 1.0186 (3) | 0.6269 (4) | 0.8847 (3) | 0.0376 (13) | |
| H6A | 1.0360 | 0.6011 | 0.8430 | 0.045* | |
| C7 | 1.0541 (2) | 0.7191 (4) | 0.9589 (3) | 0.0415 (13) | |
| H7A | 1.1002 | 0.7706 | 0.9767 | 0.050* | |
| C8 | 1.0106 (3) | 0.7288 (4) | 1.0008 (3) | 0.0444 (13) | |
| H8A | 1.0216 | 0.7865 | 1.0533 | 0.053* | |
| C9 | 0.9489 (3) | 0.6410 (4) | 0.9538 (3) | 0.0337 (12) | |
| H9A | 0.9093 | 0.6261 | 0.9681 | 0.040* | |
| C10 | 0.9534 (3) | 0.5776 (4) | 0.8825 (3) | 0.0304 (12) | |
| C11 | 0.8976 (3) | 0.4797 (4) | 0.8161 (3) | 0.0302 (12) | |
| C12 | 0.8800 (2) | 0.2468 (4) | 0.6504 (3) | 0.0272 (11) | |
| C13 | 0.8246 (2) | 0.1417 (4) | 0.5917 (3) | 0.0269 (11) | |
| C14 | 0.8305 (3) | 0.0933 (4) | 0.5195 (3) | 0.0388 (13) | |
| H14A | 0.8674 | 0.1284 | 0.5097 | 0.047* | |
| C15 | 0.7828 (3) | −0.0064 (4) | 0.4615 (3) | 0.0490 (15) | |
| H15A | 0.7875 | −0.0372 | 0.4129 | 0.059* | |
| C16 | 0.7285 (3) | −0.0601 (4) | 0.4754 (3) | 0.0422 (14) | |
| H16A | 0.6968 | −0.1283 | 0.4371 | 0.051* | |
| C17 | 0.7214 (2) | −0.0123 (4) | 0.5463 (3) | 0.0375 (13) | |
| H17A | 0.6845 | −0.0485 | 0.5556 | 0.045* | |
| C18 | 0.7675 (3) | 0.0877 (4) | 0.6038 (3) | 0.0300 (12) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Fe1 | 0.0332 (4) | 0.0339 (3) | 0.0390 (4) | 0.0019 (4) | 0.0195 (3) | −0.0003 (4) |
| O1 | 0.0258 (18) | 0.0442 (19) | 0.058 (2) | −0.0049 (17) | 0.0251 (17) | −0.0128 (15) |
| O2 | 0.0409 (17) | 0.061 (2) | 0.0440 (17) | −0.0154 (18) | 0.0328 (15) | −0.0120 (16) |
| O3 | 0.0369 (18) | 0.052 (2) | 0.0518 (19) | −0.0128 (17) | 0.0345 (16) | −0.0080 (16) |
| N1 | 0.026 (2) | 0.029 (2) | 0.043 (2) | 0.000 (2) | 0.022 (2) | −0.0085 (17) |
| N2 | 0.029 (2) | 0.038 (2) | 0.043 (2) | −0.005 (2) | 0.0246 (18) | −0.0056 (19) |
| C1 | 0.043 (3) | 0.058 (3) | 0.031 (3) | 0.006 (3) | 0.010 (3) | 0.001 (2) |
| C2 | 0.044 (3) | 0.037 (3) | 0.056 (3) | 0.013 (3) | 0.031 (3) | 0.010 (2) |
| C3 | 0.049 (3) | 0.031 (3) | 0.059 (3) | −0.007 (3) | 0.031 (3) | −0.007 (2) |
| C4 | 0.042 (3) | 0.036 (3) | 0.066 (4) | 0.002 (3) | 0.034 (3) | 0.011 (3) |
| C5 | 0.051 (3) | 0.069 (4) | 0.048 (3) | 0.019 (3) | 0.032 (3) | 0.021 (3) |
| C6 | 0.033 (3) | 0.044 (3) | 0.033 (3) | 0.010 (3) | 0.016 (2) | −0.003 (2) |
| C7 | 0.021 (2) | 0.033 (3) | 0.048 (3) | 0.011 (3) | 0.002 (2) | 0.005 (3) |
| C8 | 0.049 (3) | 0.039 (3) | 0.040 (3) | 0.000 (3) | 0.020 (3) | −0.006 (2) |
| C9 | 0.039 (3) | 0.029 (3) | 0.038 (3) | 0.002 (2) | 0.024 (2) | −0.002 (2) |
| C10 | 0.028 (3) | 0.027 (2) | 0.037 (3) | −0.001 (2) | 0.018 (2) | −0.005 (2) |
| C11 | 0.033 (3) | 0.028 (3) | 0.027 (3) | 0.001 (3) | 0.013 (2) | 0.003 (2) |
| C12 | 0.024 (2) | 0.030 (3) | 0.031 (2) | 0.005 (2) | 0.016 (2) | 0.008 (2) |
| C13 | 0.025 (3) | 0.023 (2) | 0.032 (3) | 0.000 (2) | 0.014 (2) | 0.002 (2) |
| C14 | 0.038 (3) | 0.042 (3) | 0.046 (3) | 0.000 (3) | 0.028 (3) | 0.000 (2) |
| C15 | 0.052 (4) | 0.056 (3) | 0.045 (3) | −0.006 (3) | 0.030 (3) | −0.014 (3) |
| C16 | 0.038 (3) | 0.043 (3) | 0.044 (3) | −0.017 (3) | 0.021 (3) | −0.019 (2) |
| C17 | 0.033 (3) | 0.040 (3) | 0.041 (3) | −0.012 (2) | 0.020 (3) | −0.007 (2) |
| C18 | 0.034 (3) | 0.030 (3) | 0.031 (3) | 0.005 (2) | 0.020 (2) | 0.002 (2) |
Geometric parameters (Å, °)
| Fe1—C2 | 2.008 (4) | C3—H3A | 0.9800 |
| Fe1—C9 | 2.013 (4) | C4—C5 | 1.405 (6) |
| Fe1—C10 | 2.014 (4) | C4—H4A | 0.9800 |
| Fe1—C1 | 2.021 (4) | C5—H5A | 0.9800 |
| Fe1—C5 | 2.028 (5) | C6—C7 | 1.412 (5) |
| Fe1—C6 | 2.031 (4) | C6—C10 | 1.416 (6) |
| Fe1—C3 | 2.031 (4) | C6—H6A | 0.9800 |
| Fe1—C8 | 2.031 (4) | C7—C8 | 1.412 (6) |
| Fe1—C4 | 2.033 (4) | C7—H7A | 0.9800 |
| Fe1—C7 | 2.045 (4) | C8—C9 | 1.400 (5) |
| O1—C11 | 1.240 (5) | C8—H8A | 0.9800 |
| O2—C12 | 1.233 (4) | C9—C10 | 1.410 (5) |
| O3—C18 | 1.349 (5) | C9—H9A | 0.9800 |
| O3—H3B | 0.822 (10) | C10—C11 | 1.478 (5) |
| N1—C11 | 1.340 (5) | C12—C13 | 1.484 (5) |
| N1—N2 | 1.381 (4) | C13—C14 | 1.379 (6) |
| N1—H1B | 0.871 (10) | C13—C18 | 1.407 (6) |
| N2—C12 | 1.343 (5) | C14—C15 | 1.381 (5) |
| N2—H2B | 0.860 (10) | C14—H14A | 0.9300 |
| C1—C5 | 1.402 (6) | C15—C16 | 1.373 (6) |
| C1—C2 | 1.404 (6) | C15—H15A | 0.9300 |
| C1—H1A | 0.9800 | C16—C17 | 1.370 (6) |
| C2—C3 | 1.403 (6) | C16—H16A | 0.9300 |
| C2—H2A | 0.9800 | C17—C18 | 1.369 (5) |
| C3—C4 | 1.410 (6) | C17—H17A | 0.9300 |
| C2—Fe1—C9 | 105.43 (18) | C4—C3—H3A | 126.6 |
| C2—Fe1—C10 | 121.17 (19) | Fe1—C3—H3A | 126.6 |
| C9—Fe1—C10 | 41.01 (16) | C5—C4—C3 | 109.2 (4) |
| C2—Fe1—C1 | 40.79 (16) | C5—C4—Fe1 | 69.5 (3) |
| C9—Fe1—C1 | 122.31 (19) | C3—C4—Fe1 | 69.6 (3) |
| C10—Fe1—C1 | 107.34 (18) | C5—C4—H4A | 125.4 |
| C2—Fe1—C5 | 68.7 (2) | C3—C4—H4A | 125.4 |
| C9—Fe1—C5 | 159.32 (19) | Fe1—C4—H4A | 125.4 |
| C10—Fe1—C5 | 123.77 (19) | C1—C5—C4 | 107.0 (5) |
| C1—Fe1—C5 | 40.53 (17) | C1—C5—Fe1 | 69.5 (3) |
| C2—Fe1—C6 | 158.24 (18) | C4—C5—Fe1 | 70.0 (3) |
| C9—Fe1—C6 | 68.96 (18) | C1—C5—H5A | 126.5 |
| C10—Fe1—C6 | 40.98 (16) | C4—C5—H5A | 126.5 |
| C1—Fe1—C6 | 123.23 (18) | Fe1—C5—H5A | 126.5 |
| C5—Fe1—C6 | 108.72 (19) | C7—C6—C10 | 107.0 (4) |
| C2—Fe1—C3 | 40.66 (16) | C7—C6—Fe1 | 70.3 (2) |
| C9—Fe1—C3 | 120.45 (18) | C10—C6—Fe1 | 68.9 (2) |
| C10—Fe1—C3 | 156.7 (2) | C7—C6—H6A | 126.5 |
| C1—Fe1—C3 | 68.42 (18) | C10—C6—H6A | 126.5 |
| C5—Fe1—C3 | 68.83 (19) | Fe1—C6—H6A | 126.5 |
| C6—Fe1—C3 | 160.36 (19) | C8—C7—C6 | 108.7 (4) |
| C2—Fe1—C8 | 121.38 (19) | C8—C7—Fe1 | 69.2 (2) |
| C9—Fe1—C8 | 40.51 (16) | C6—C7—Fe1 | 69.2 (2) |
| C10—Fe1—C8 | 68.62 (17) | C8—C7—H7A | 125.6 |
| C1—Fe1—C8 | 158.0 (2) | C6—C7—H7A | 125.6 |
| C5—Fe1—C8 | 159.5 (2) | Fe1—C7—H7A | 125.6 |
| C6—Fe1—C8 | 68.81 (18) | C9—C8—C7 | 107.6 (4) |
| C3—Fe1—C8 | 106.29 (19) | C9—C8—Fe1 | 69.0 (2) |
| C2—Fe1—C4 | 67.96 (18) | C7—C8—Fe1 | 70.2 (2) |
| C9—Fe1—C4 | 157.47 (19) | C9—C8—H8A | 126.2 |
| C10—Fe1—C4 | 160.9 (2) | C7—C8—H8A | 126.2 |
| C1—Fe1—C4 | 67.63 (19) | Fe1—C8—H8A | 126.2 |
| C5—Fe1—C4 | 40.48 (17) | C8—C9—C10 | 108.4 (4) |
| C6—Fe1—C4 | 125.03 (19) | C8—C9—Fe1 | 70.5 (2) |
| C3—Fe1—C4 | 40.59 (17) | C10—C9—Fe1 | 69.5 (2) |
| C8—Fe1—C4 | 123.09 (19) | C8—C9—H9A | 125.8 |
| C2—Fe1—C7 | 158.59 (19) | C10—C9—H9A | 125.8 |
| C9—Fe1—C7 | 68.03 (18) | Fe1—C9—H9A | 125.8 |
| C10—Fe1—C7 | 68.14 (18) | C9—C10—C6 | 108.2 (4) |
| C1—Fe1—C7 | 159.89 (19) | C9—C10—C11 | 124.8 (4) |
| C5—Fe1—C7 | 124.4 (2) | C6—C10—C11 | 127.0 (4) |
| C6—Fe1—C7 | 40.55 (15) | C9—C10—Fe1 | 69.5 (2) |
| C3—Fe1—C7 | 123.62 (18) | C6—C10—Fe1 | 70.2 (2) |
| C8—Fe1—C7 | 40.54 (16) | C11—C10—Fe1 | 124.7 (3) |
| C4—Fe1—C7 | 109.83 (19) | O1—C11—N1 | 121.9 (4) |
| C18—O3—H3B | 120 (3) | O1—C11—C10 | 122.7 (4) |
| C11—N1—N2 | 116.7 (4) | N1—C11—C10 | 115.3 (4) |
| C11—N1—H1B | 128 (2) | O2—C12—N2 | 120.7 (4) |
| N2—N1—H1B | 114 (2) | O2—C12—C13 | 121.8 (4) |
| C12—N2—N1 | 120.5 (3) | N2—C12—C13 | 117.6 (4) |
| C12—N2—H2B | 119 (2) | C14—C13—C18 | 117.9 (4) |
| N1—N2—H2B | 120 (2) | C14—C13—C12 | 116.4 (4) |
| C5—C1—C2 | 108.5 (4) | C18—C13—C12 | 125.7 (4) |
| C5—C1—Fe1 | 70.0 (3) | C13—C14—C15 | 121.4 (5) |
| C2—C1—Fe1 | 69.1 (3) | C13—C14—H14A | 119.3 |
| C5—C1—H1A | 125.7 | C15—C14—H14A | 119.3 |
| C2—C1—H1A | 125.7 | C16—C15—C14 | 120.1 (5) |
| Fe1—C1—H1A | 125.7 | C16—C15—H15A | 120.0 |
| C3—C2—C1 | 108.5 (4) | C14—C15—H15A | 120.0 |
| C3—C2—Fe1 | 70.6 (3) | C17—C16—C15 | 119.3 (4) |
| C1—C2—Fe1 | 70.1 (3) | C17—C16—H16A | 120.3 |
| C3—C2—H2A | 125.8 | C15—C16—H16A | 120.3 |
| C1—C2—H2A | 125.8 | C18—C17—C16 | 121.4 (4) |
| Fe1—C2—H2A | 125.8 | C18—C17—H17A | 119.3 |
| C2—C3—C4 | 106.8 (4) | C16—C17—H17A | 119.3 |
| C2—C3—Fe1 | 68.8 (2) | O3—C18—C17 | 120.8 (4) |
| C4—C3—Fe1 | 69.8 (3) | O3—C18—C13 | 119.2 (4) |
| C2—C3—H3A | 126.6 | C17—C18—C13 | 120.0 (4) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H2B···O3 | 0.860 (10) | 1.95 (2) | 2.631 (4) | 135 (3) |
| O3—H3B···O1i | 0.822 (10) | 1.908 (15) | 2.705 (4) | 163 (4) |
| N1—H1B···O2ii | 0.871 (10) | 2.03 (2) | 2.810 (4) | 148 (4) |
Symmetry codes: (i) −x+3/2, y−1/2, −z+3/2; (ii) −x+2, y, −z+3/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BH2153).
References
- Beer, P. D. (1992). Adv. Inorg. Chem.39, 79–157.
- Beer, P. D. & Smith, D. K. (1997). Prog. Inorg. Chem.46, 1–8.
- Bruker (2000). SMART (Version 5.054), SAINT-Plus (Version 6.22) and SADABS (Version 2.03). Bruker AXS Inc., Madison, Wisconsin, USA.
- Long, N. J. (1995). Angew. Chem. Int. Ed. Engl.34, 21–38.
- Miller, J. S. & Epstein, A. J. (1994). Angew. Chem. Int. Ed. Engl.33, 385–415.
- Nguyen, P., Gómez-Elipe, P. & Manners, I. (1999). Chem. Rev.99, 1515–1548. [DOI] [PubMed]
- Sheldrick, G. M. (1997a). SHELXS97 and SHELXL97 University of Göttingen, Germany.
- Sheldrick, G. M. (1997b). SHELXTL Bruker AXS Inc., Madison, Wisconsin, USA.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536807065634/bh2153sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536807065634/bh2153Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report