Figure 1. Phosphorylation of NKCC1 by AMPK and identification of the sites.
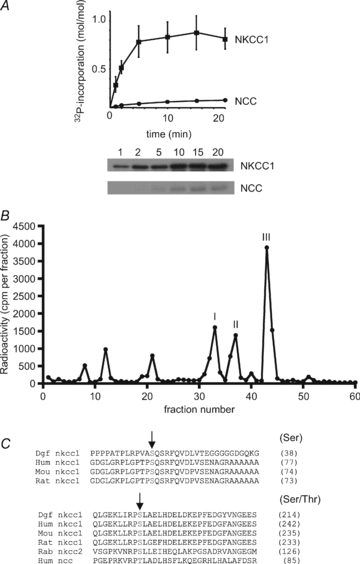
A, recombinant GST-dogfish NKCC1 and GST-human NCC were phosphorylated in vitro with AMPK and [γ-32P]MgATP. At the indicated times, aliquots were removed for SDS-PAGE and phosphorimaging for the measurement of 32P incorporation. The results are the means ±s.e.m. of three separate experiments and representative autoradiograms are shown below. B, after maximal in vitro phosphorylation by AMPK, GST-dogfish NKCC1 was precipitated and digested with trypsin. Peptides were separated by reverse-phase narrow-bore HPLC in an acetonitrile gradient and fractions were counted by Cerenkov radiation. Phosphorylation sites in the three radiolabelled peaks were identified by mass spectrometry. C, an alignment of N-terminal domain sequences surrounding the AMPK sites of dogfish NKCC1 with human, mouse and rat, as well as with sequences of rabbit NKCC2 and human NCC is shown. AMPK phosphorylation sites in dogfish NKCC1 are indicated by arrows and numbers between brackets refer to the residues corresponding to dogfish Ser38 and Ser214 in the different species.
