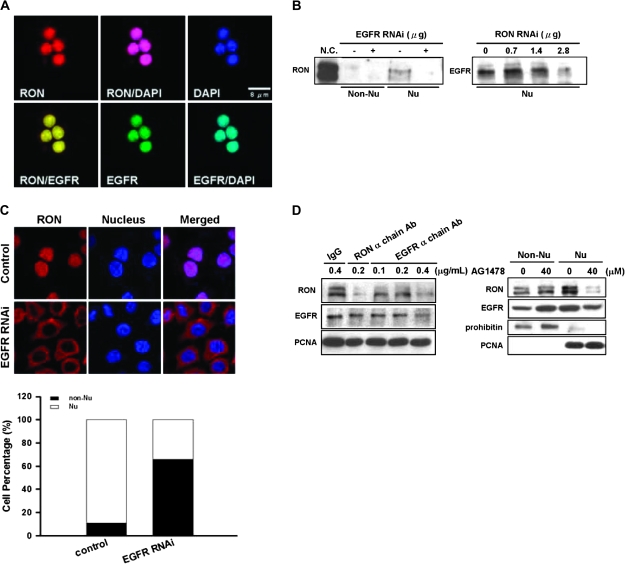
Fig. 3.
Interactions between EGFR and nuclear RON in bladder cancer cells. (A) Immunofluorescence analysis showed that, upon 24 h of serum starvation, RON (red) was colocalized with EGFR (green) in the nucleus of TSFG8301 cells. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (blue) and images acquired in an Olympus Confocal Microscope. (B) Nuclear RON expression was reduced by EGFR siRNA in TSGH8301 cells. After 48 h of either EGFR (left) or RON (right) siRNA transfection, nuclear extracts were collected for immunoblotting analysis. (C) RON (red) location was redistributed upon attenuating EGFR expression by EGFR siRNA. After 48 h transfection of EGFR siRNA, TSGH8301 cells were subjected to immunofluorescence analysis. Nuclei were counterstained with DAPI (blue). Staining intensities were quantified and expressed as histogram (bottom panel). (D) TSGH8301 cells were treated with indicated doses of EGFR neutralization antibody (left panel; lanes 3–5) and nuclear extracts were collected for western blot analysis. Treatment of RON α chain antibody (lane 3) was utilized as a positive control, whereas normal IgG antibody (lane 1) was used as a negative control to the neutralization antibodies. In ‘right panel’, cells were pretreated with optimized doses of AG1478, an EGFR-specific inhibitor, and then serum-starved for 24 h. Subcellular fractions were collected for immunoblotting with antibodies as indicated.