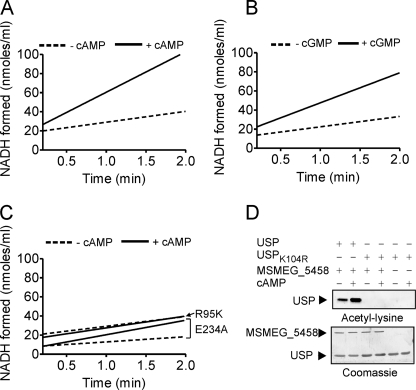
FIGURE 5.
Initial rates of the acetyltransferase activity of MSMEG_5458. The acetyltransferase activities of wild type (A and B) and mutant MSMEG_5458 proteins (C) were measured using the coupled assay. MSMEG_5458 or mutant proteins (1 μg) were assayed in the presence of 30 μm acetyl-CoA and 50 μm USP. The initial rate of formation of NADH is shown, after subtracting the change in absorbance at 340 nm that is seen in assays performed in the absence of the enzymes, which was usually ∼1% of the change seen in the presence of enzyme. D, USPK104R (50 μm) was used as substrate for the acetyltransferase activity, in the presence or absence of cAMP (10 μm). Samples were subjected to Western blotting using acetyl-lysine antibodies, and either wild-type USP or USPK104R. Data shown are a representative of assays performed thrice.