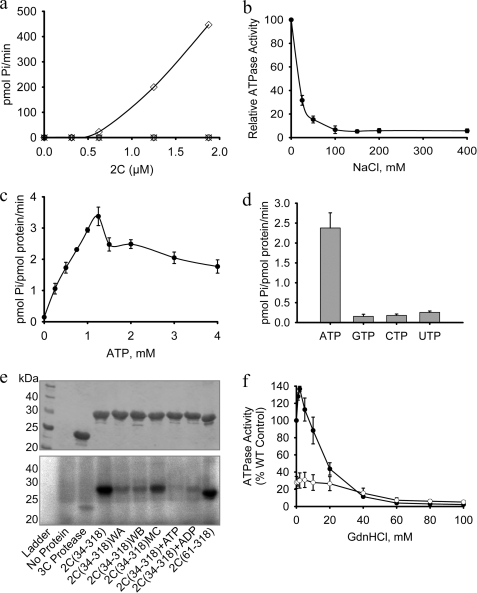
FIGURE 2.
FMDV 2C is a specific ATPase that is sensitive to NaCl and GdnHCl. The ATPase activity of FMDV 2C(34–318) was measured in 100-μl reactions containing 4 μg of protein (1.25 μm), 1 mm ATP, and 2 mm MgCl2, unless otherwise stated. a, ATP hydrolysis was measured in the presence of varying concentrations of protein (open diamonds). There was no detectable ATPase activity for the Walker A, K116A (WA) (open circles), Walker B, D160A (WB) (open triangles), and Motif C, N207A (MC) (crosses). The data plotted are an average of two experiments. b, the ATPase activity is very sensitive to NaCl and decreases monotonically with an ED50 of about 10 mm. c, the ATPase activity of 2C(34–318) varies in a complex manner with increases in ATP concentration, exhibiting a sharp decline between 1.25 and 1.5 mm substrate. d, comparative NTPase activity of 2C(34–318) with 1 mm each ATP, GTP, CTP, and UTP. e, UV cross-linking of [α-32P]ATP to FMDV 2C(34–318) and various mutants (identified below each lane). After cross-linking, the protein sample was run on a 12% SDS-polyacrylamide gel. Top, the positions of protein bands were confirmed by staining with Coomassie Blue before gel drying; bottom, cross-linked ATP was detected by exposure to a phosphor screen (bottom). 5 mm unlabeled ADP or ATP was included as indicated. f, effect of GdnHCl on the ATPase activity of wild-type FMDV 2C(34–318) (WT) (closed circles) and the 2C(34–318) M159L mutant (open circles). Error bars, S.E. determined from three independent measurements. WA, Walker A; WB, Walker B; MC, motif C.