Abstract
Bile acid deficiency is a serious syndrome in newborns that can result in death if untreated. 5β-Reductase deficiency is one form of bile acid deficiency and is characterized by dramatically decreased levels of physiologically active 5β-reduced bile acids. AKR1D1 (aldo-keto reductase 1D1) is the only known human enzyme that stereo-specifically reduces the Δ4 double bond in 3-keto steroids and sterols to yield the 5β-hydrogenated product. Analysis of the AKR1D1 gene in five patients with 5β-reductase deficiency revealed five different mutations resulting in an amino acid substitution in the protein. To investigate a causal role for these observed point mutations in AKR1D1 in 5β-reductase deficiency, we characterized their effect on enzymatic properties. Attempts to purify mutant enzymes by overexpression in Escherichia coli only yielded sufficient amounts of the P133R mutant for further characterization. This enzyme displayed a highly reduced Km and Vmax reminiscent of uncompetitive kinetics with 4-cholesten-7α-ol-3-one as substrate. In addition, this mutant displayed no change in cofactor affinity but was more thermolabile in the absence of NADPH as judged by CD spectroscopy. All mutants were compared following expression in HEK 293 cells. Although these enzymes were equally expressed based on mRNA levels, protein expression and functional activity were dramatically reduced. Cycloheximide treatment also revealed that several of the expressed mutants were less stable. Our findings show that the reported mutations in AKR1D1 in patients with 5β-reductase lead to significantly decreased levels of active enzyme and could be causal in the development of bile acid deficiency syndrome.
Keywords: Bile Acid, Enzyme Kinetics, Mutant, Protein Stability, Steroid, 5beta-Reduction, Aldo-keto Reductase
Introduction
AKR1D1 (aldo-keto reductase 1D1) is implicated as one of the key enzymes in bile acid biosynthesis. The enzyme is the only known human Δ4-3-ketosteroid 5β-reductase and catalyzes the reduction of the Δ4-3-ketosteroid to form the A/B cis ring structure, utilizing NADPH as a cofactor (1). The Δ4-3-ketosteroid functionality is common to all steroid hormones except the estrogens, and the C4-C5 double bond can be further reduced in a stereo-specific manner. 5α-Reduction of testosterone to 5α-dihydrotestosterone results in increased androgen receptor activation (2, 3), whereas 5β-reduction of progesterone to 5β-pregnane-3,20-dione results in activation of the pregnane X receptor (4) and constitutive active/androstane receptor (5). In bile acid biosynthesis, AKR1D1 reduces Δ4-cholesten-7α-ol-3-one and Δ4-cholesten-7α,12α-diol-3-one to their respective 5β-dihydrosteroid forms (6). The resulting 5β-reduced structure contains a 90° bend in the steroid scaffold that is believed to generate the essential emulsification characteristics of the resultant human bile acids.
Human 5β-reductase deficiency (OMIM 604741) was first diagnosed by Setchell et al. (7) in siblings with neonatal hepatitis and cholestatis. Since then, more than 20 cases have been reported, characterized by reduced primary bile acid biosynthesis and accumulation of hepatotoxic Δ4-3-oxo- and 5α-reduced (allo-) bile acids (8–11). The deficiency can be treated with primary bile acids (12, 13), which normalize liver morphology and return liver function to normal. This treatment serves two functions. First there is feedback repression of the 7α-hydroxylase gene (CYP7A1), the key regulatory and rate-limiting step in primary bile acid biosynthesis, and this prevents the accumulation of the deleterious Δ4-3-one precursors and allo-bile acids (14). Second, the natural bile acids administered lead to normal emulsification of fat and absorption of fat-soluble vitamins. Several point mutations in AKR1D1 (L106F, P133R, P198L, G223E, and R261C) have been detected in patients with bile acid deficiency; however, the effects of the observed mutations on enzyme structure-function and whether they are causal in the observed phenotype have remained unclear (8, 10, 15).
Recently, knowledge about AKR1D1 enzyme function increased with the elucidation of its crystal structure in complex with cofactor and different steroid substrates and products (16, 17). These studies revealed that AKR1D1 had an (α/β)8-barrel structure with three large loops (A, B, and C) at the back of the barrel and contained similar cofactor and steroid substrate binding sites compared with other AKR1C enzymes. These enzymes, AKR1C1–AKR1C4, act as 3-, 17-, and 20-ketosteroid reductases and have been thoroughly characterized (18–22). The amino acids of the aldo-keto reductase catalytic tetrad, consisting of Asp50, Tyr55, Lys84, and His117 (numbering according to rat AKR1C9 (3α-hydroxysteroid dehydrogenase)) are highly conserved in AKR1D1. However, substitution of histidine by glutamatic acid translates into a functional switch from ketosteroid reduction (in AKR1C enzymes) to double bond reduction (in AKR1D1) (23).
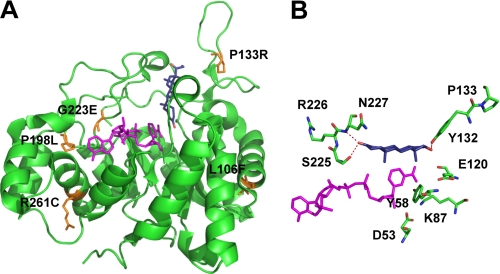
Examination of the AKR1D1 crystal structure allowed us to map the position of the reported point mutations associated with bile acid deficiency (Fig. 1A). We found that all mutations except for P133R reside in areas highly conserved across AKR1D1 homologs in other mammalian species. However, none of the positions are in direct contact with the catalytic tetrad, cofactor, or substrate binding sites, and therefore it remains unclear whether they could indeed be causal in the reported cases of bile acid deficiency.
FIGURE 1.
Location of the disease-related mutants in the AKR1D1 crystal structure. A, position of the mutants on the structure and their relationship to cofactor (magenta) and steroid (blue) binding. B, a perspective view of the location of Pro133 and its relationship to binding testosterone in the unproductive binding mode.
In this study, we examined the effects of the observed point mutations on enzyme function following their introduction into wild type AKR1D1. We report the expression, purification, and characterization of AKR1D1-P133R and compare it with the wild type enzyme. Furthermore, although enzymes with the point mutations L106F, P198L, G223E, and R261C could not be purified, we were able to study these enzymes following expression in mammalian cells. Our results indicate that all four point mutations had severe effects on one or more of the following: steady state kinetic parameters, enzyme stability, and amount of soluble protein expressed in cells. In summary, although none of the observed mutations affects catalysis in AKR1D1 directly, all drastically reduced 5β-reductase activity in a biological context. Hence, all AKR1D1 mutations reported in patients with bile acid deficiency and characterized in this study have the potential to cause the observed phenotype of 5β-reductase deficiency.
EXPERIMENTAL PROCEDURES
Materials
The vector pET-16b was purchased from Novagen. The GeneAmp RNA PCR Core kit was purchased from PerkinElmer Life Sciences. Escherichia coli strain C41 (DE3) was provided by Dr. J. E. Walker (Medical Research Council Laboratory of Molecular Biology, Cambridge, UK). The QuikChange II site-directed mutagenesis kit was purchased from Stratagene. Restriction endonucleases were purchased from New England Biolabs. Synthetic oligonucleotides were obtained from Invitrogen. NADPH was obtained from Roche Applied Science. Steroids were purchased from Steraloids, Inc. [4-14C]Testosterone (50 mCi/mmol) was obtained from PerkinElmer Life Sciences. Nickel-Sepharose 6 Fast Flow was purchased from Amersham Biosciences. The bovine serum albumin protein standard was purchased from Sigma. Bradford reagent and Restore Western blot stripping buffer were purchased from Bio-Rad. Antibodies were obtained from GE Healthcare (ECL anti-mouse IgG from sheep), Sigma (anti-β-actin), or Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) (horseradish peroxidase-conjugated anti-rabbit from mouse). All cell culture reagents except for fetal calf serum (Thermo Scientific) were purchased from Invitrogen. HEK 293 cells were obtained from ATCC. All other reagents were of ACS quality (i.e. they meet the specifications of the American Chemical Society) or higher.
Construction of Expression Vectors
Previously, we reported the expression of AKR1D1 using the prokaryotic expression vectors pET16b and pET28a (16). For expression in HEK 293 cells, the AKR1D1 coding sequence was subcloned into pcDNA3.1 vector from a modified pET16b construct lacking the His tag using the compatible XbaI and BamHI restriction sites. Point mutations were subsequently introduced by site-directed mutagenesis using the QuikChange method and the following forward and reverse primer pairs: L106F, 5′-dCCC TGG AGA GGA CAT TCA GGG TCC TCC AGC-3′ and 5′-dGCT GGA GGA CCC TGA ATG TCC TCT CCA GGG-3′; P133R, 5′-dGCC AGG AGA TGA AAT ATA CCG TAG AGA TGA GAA TGG C-3′ and 5′-dGCC ATT CTC ATC TCT ACG GTA TAT TTC ATC TCC TGG C-3′; P198L, 5′-dCCA GGT TGA GTG CCA TCT GTA TTT CAC CCA GCC-3′ and 5′-dGGC TGG GTG AAA TAC AGA TGG CAC TCA ACC TGG-3′; G223E, 5′-dGCA TAT AGC CCT TTG GAG ACC AGT AGG AAT CC-3′ and 5′-dGGA TTC CTA CTG GTC TCC AAA GGG CTA TAT GC-3′; R261C, 5′-dGCA GCT CAA ATT GTT TTG TGT TTC AAC ATC CAG CGA GGG-3′ and 5′-dCCC TCG CTG GAT GTT GAA ACA CAA AAC AAT TTG AGC TGC-3′. Mutated codons are indicated by the underlined nucleotides. All constructs were verified by dideoxy sequencing.
Expression and Purification of AKR1D1-P133R
Recombinant AKR1D1-P133R was purified to homogeneity following a protocol recently established for the wild type enzyme (16). AKR1D1-P133R was obtained in 16% yield and had a final specific activity of 24 nmol of testosterone reduced min−1 mg−1 of purified enzyme under standard radiometric assay conditions as described recently (24). Homogeneity of the AKR1D1-P133R mutant enzyme was checked by SDS-PAGE. Protein concentration was measured using the Bradford assay, according to the manufacturer's instructions, using bovine serum albumin as a standard.
Measurement of Steady State Kinetic Parameters by Spectrofluorimetric Assay
The kcat and Km values for testosterone and cortisone were determined fluorimetrically as described before (24) with steroid substrate concentration ranging from 0.60 to 40 μm. Kinetic analyses of initial velocities obtained were performed by fitting the data using the program GraFit to the Henri-Michaelis-Menten equation,
where v is the initial velocity of the reaction, [S] is the molar concentration of the substrate, and Km is the Michaelis-Menten constant for the substrate. Dividing Vmax by the molar concentration of the enzyme gave kcat. When substrate inhibition was observed, initial velocity data were fit in a similar manner to the following equation,
where variables were the same as in Equation 1 with the inclusion of Ki, which is the dissociation constant for substrate from the E·NADP+ complex.
Fluorescence Titration with NADPH
The dissociation constant (Kd) of the enzyme for NADPH was determined by monitoring the quenching of the intrinsic protein fluorescence upon the addition of cofactor as described (25) with the following changes. Titrations were performed in 10 mm potassium phosphate (pH 6.0) in a 1.4-ml volume at 37 °C using 0.11 μm AKR1D1-P133R. Emission spectra were monitored from 320 to 500 nm on a fluorescence spectrophotometer F-4500 after excitation at 295 nm. The data were fitted to the Morrison equation (26) (using Equations 3 and 4) to compensate for the similar concentration of enzyme and ligand in the titration and determine the dissociation constant,
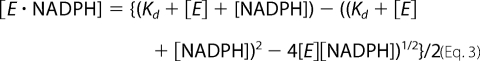 |
where [E·NADPH] is the concentration of E·NADPH binary complex, [E] is the total concentration of enzyme, ΔF is the difference in fluorescence in the presence and absence of NADPH, and ΔFmax is the maximum ΔF at the saturating concentration of NADPH.
Circular Dichroism Experiments
Circular dichroism spectropolarimetry was performed with wild type (4.8 × 10−6 m) and AKR1D1-P133R (3.8 × 10−6 m) in 10 mm potassium phosphate (pH 7.0). Protein spectra were recorded between a 260- and 190-nm wavelength at 22 °C in millidegrees on a Jasco J810 spectropolarimeter in a 0.1-cm cell. A background spectrum was obtained with only buffer. Following the spectral scans, melt curves were performed on each of the samples at 222 nm by raising the temperature from 0 to 90 °C in increments of 2 °C. A second protein spectrum was recorded for each sample after the melt curve was complete.
Heat Stability Experiments
To determine the thermal stability of the enzymes, wild type and AKR1D1-P133R were heated for 10 min at various increments from 25 to 50 °C in 10 mm potassium phosphate (pH 7.0) and 1 mm EDTA. Samples were placed on ice, centrifuged for 1 min to remove any precipitate, and then assayed fluorimetrically.
Cell Culture and Transient Transfection
HEK 293 cells were maintained in 10-cm culture dishes at 37 °C and 5% CO2 in Dulbecco's modified Eagle's medium containing GlutaMAXTM and supplemented with 10% heat-inactivated fetal calf serum and 1% penicillin, streptomycin. For experiments, 1 million cells/well were seeded into 6-well plates and the following day were transfected with either 1.5 μg of the respective AKR1D1 construct or empty vector per well using FuGene 6 according to the manufacturer's protocol. Forty-eight hours later, cells were either harvested for RNA expression and analysis of enzyme activity or subjected to cycloheximide treatment.
Cycloheximide Treatment
Cells were seeded and transiently transfected as described above. Forty-eight hours following transfection, cells were harvested by scraping (0 h time point), or cycloheximide (dissolved in water and sterile filtered) was added to the medium to a final concentration of 20 μg/ml, and cells were harvested 0, 6, and 24 h following treatment.
Reverse Transcription-PCR
RNA was extracted from cells using the RNeasy minikit from Qiagen according to the manufacturer's protocol. To obtain cDNA, 1 μg of RNA was reverse transcribed with random hexamer primers by use of the GeneAmp RNA PCR core kit (Applied Biosystems). Quality of the cDNA was monitored by PCR amplification of GAPDH (primers 5′-dCAT CTC TGC CCC CTC TGC TGA-3′ and 5′-dGGA TGA CCT TGC CCA CAG CCT-3′). Endogenous AKR1D1 was amplified with a forward primer matching a sequence in exon 7 (5′-dGGG GTG GTT GTC ATT CCT AA-3′) and a reverse primer matching a sequence in the 3′-untranslated region of the gene (5′-dGAC TAC CCA TTG CAC CGT CT-3′). For exogenous AKR1D1 detection, the same forward primer was employed together with a reverse primer that anneals in the cloning vector 3′ of the insert (5′-dAAC TAG AAG GCA CAG TCG AG-3′).
Polyclonal AKR1D1 Antibody
A rabbit polyclonal antiserum raised against full-length purified His-tagged AKR1D1 was produced by ProSci, Inc. (Poway, CA). Antiserum preparation from the first bleed showed the lowest cross-reactivity for the detection of other human aldo-keto reductases and was subjected to further purification to improve specificity against AKR1D1. For this, 50–150 μg of human AKR1A1 and AKR1C1 to AKR1C4 were blotted onto a strip of nitrocellulose membrane. This membrane was incubated with a 1:50 dilution of the antiserum in Tris-buffered saline (TBS)3 containing 0.1% Tween 20 (TBST) for 1 h at 4 °C. Diluted antiserum was removed, the membrane was stripped for 20 min at 42 °C and washed three times 10 min each with TBS at room temperature, and the diluted antiserum from before was reapplied. This cycle was repeated seven times, and the precleaned polyclonal α-AKR1D1 antibody was diluted 1:50 with TBS and then mixed 1:1 with glycerol and 0.02% sodium azide (final concentration) before aliquoting and storage at −20 °C. Enhanced specificity of this antibody for AKR1D1 versus a panel of human AKRs is documented in supplemental Fig. S1.
Western Blot
Cell pellets were washed once in Dulbecco's phosphate-buffered saline and then lysed by incubation in lysis buffer (20 mm Tris-HCl, pH 8.0, 150 mm NaCl, 10% glycerol, 1% Nonidet P-40, 5 mm EDTA, 0.5 mm EGTA, 20 mm β-glycerophosphate, 100 μm sodium orthovanadate, 1× proteinase inhibitor mix (Roche Applied Science)) on ice for 30 min with intermittent agitation. Lysates were centrifuged, the soluble fraction was removed, and the protein concentration was measured by a Bradford assay. Eighty micrograms of total protein per sample well were resolved by SDS-PAGE and blotted onto a nitrocellulose membrane. For Western blot development, the membrane was blocked in 5% dry milk in TBST, the polyclonal anti-AKR1D1 antibody was diluted to 1:2000 in 3% dry milk in TBST, the secondary antibody (monoclonal mouse anti-rabbit horseradish peroxidase conjugate, Santa Cruz Biotechnology, Inc.) was diluted to 1:10,000 in 3% dry milk in TBST, and the signals were visualized using the ECL technique (GE Healthcare). To detect the mutants that were poorly expressed, the secondary antibody was diluted 1:100,000 in 3% dry milk in TBST, and the signals were visualized using the SuperSignal West Femto Kit (Thermo Scientific). For the subsequent detection of β-actin, blots were stripped by incubating twice for 10 min in 42 °C Western stripping buffer (Bio-Rad) and washing three times for 10 min each in TBST, and the procedure was then completed as described above. The primary antibody monoclonal mouse anti-β-actin was applied in a 1:1000 dilution in 3% dry milk in TBST, and the secondary antibody sheep anti-mouse horseradish peroxidase conjugate was diluted 1:5000 in 3% dry milk in TBST. Detection was achieved by use of the ECL technique (GE Healthcare).
Detection of 5β-Reductase Activity in HEK 293 Cells
Cells were seeded and transiently transfected as described above. At 46 h after transfection, medium was replaced with 2 ml of Dulbecco's modified Eagle's medium containing 1% heat-inactivated fetal calf serum, GlutaMAXTM, and 1% penicillin, streptomycin per well. Two hours later, testosterone was added in 10 μl of DMSO to yield final concentrations ranging from 0.5 to 20 μm per well. Final concentrations below 10 μm contained 20 nCi, and higher final concentrations contained 30 nCi of 14C-labeled testosterone, respectively. At time points, 200 μl of medium was removed and extracted twice with 2.5 volumes of cold water-saturated ethyl acetate, and the combined organic phase was dried down. Recovery of radioactive material following extraction was >90%.
Product Identification by Thin Layer Radiochromatography
Dried samples were resuspended in ethyl acetate, applied to Partisil LK6D Silica TLC (thin layer chromatography) plates (Whatman International Ltd.), and developed twice in toluene/acetone (80:20 v/v). Radiochromatograms were scanned with an automatic TLC-linear analyzer (Bioscan Imaging Scanner System 200-IBM with AutoChanger 3000, Bioscan (Washington, D. C.)), and the relative percentage of peaks was compared and quantified as nmol of product formed using the specific radioactivity of the isotope assuming that each steroid was recovered with the same efficiency. Products were identified by co-migration with authentic standards applied to the same plate following visualization by spraying with acetic acid/sulfuric acid/anisaldehyde (100:2:1, v/v/v) and heat.
RESULTS
Expression and Purification of Recombinant Disease-related AKR1D1 Mutants
To examine whether the disease-related AKR1D1 mutants could account for bile acid deficiency, we set out to purify the L106F, P133R, P198L, and R261C enzymes and compare their properties with wild type AKR1D1. Based on SDS-polyacrylamide gels, all four mutants could be clearly detected in bacterial sonicates following overnight induction of the expression of the His-tagged proteins in E. coli (data not shown). However, the majority of the expressed mutants accumulated in inclusion bodies. Attempts to purify the disease-related mutants resulted in extremely low yields, and only the AKR1D1-P133R mutant could be purified in milligram amounts to homogeneity for further analysis (supplemental Table S1). Subsequent to this portion of the study, the AKR1D1-G223E mutant was described (15), and this mutant was compared in mammalian transfection studies with the other disease-based mutants (see below).
Comparison of Kinetic Constants between Wild Type and AKR1D1-P133R
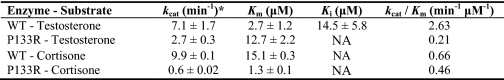
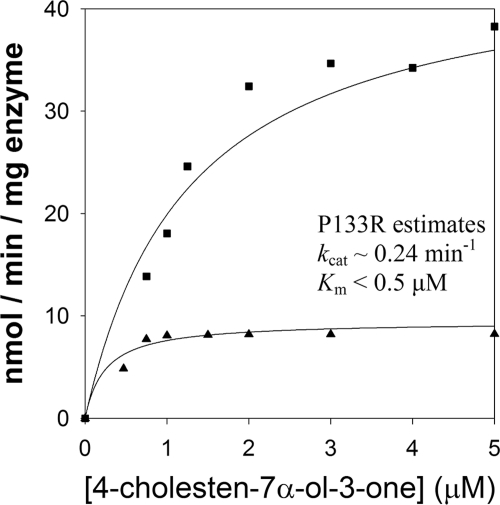
The reported P133R mutation is present in loop A of the enzyme and is not directly involved in forming the protein scaffold or the catalytic tetrad (16, 17) (Fig. 1A). We determined steady state kinetic constants for the wild type and AKR1D1-P133R mutant. The specific activity of the homogenous recombinant mutant enzyme with 10 μm testosterone was 24 nmol min−1 mg−1 and was 3-fold lower than wild type enzyme. The catalytic efficiency toward testosterone (0.21 min−1 μm−1) was found to be 12-fold less as reflected by an increase in Km (12.7 μm) and a decrease in kcat (2.7 min−1) (Table 1). Interestingly, the substrate inhibition of wild type enzyme by testosterone was not observed with the P133R mutant. By contrast, Δ4-3-ketosteroids with longer C17 side chains (e.g. cortisone and Δ4-cholesten-7α-ol-3-one) showed large decreases in kcat and Km. The kcat of cortisone was decreased 16-fold (0.6 min−1), and Km was decreased 12-fold (1.3 μm). The same pattern was observed with Δ4-cholesten-7α-ol-3-one; however, it was not possible to accurately measure the Km for this substrate because the enzyme was essentially saturated at the lowest concentrations of substrate at which a reaction rate could be reliably measured (Fig. 2). At saturation, kcat for Δ4-cholesten-7α-ol-3-one was depressed 7-fold. By contrast, Km is significantly lower than 0.8 μm, the value for wild type enzyme. Thus, the effect of this mutation was a change from a low affinity, high capacity enzyme to a high affinity, low capacity enzyme.
TABLE 1.
Comparison of kinetic constants of wild type AKR1D1 and the AKR1D1-P133R mutant
NA, not applicable (not observed).
* All activity measurements were determined fluorimetrically.
FIGURE 2.
Comparison of velocity versus substrate plots for wild type AKR1D1 and the P133R mutant using Δ4-cholesten-7α-ol-3-one as substrate. ■, AKR1D1; ▴, P133R. Assays were performed using standard fluorimetric assay conditions. For the wild type enzyme, the experiment was replicated twice, and for the P133R mutant, the experiment was replicated once. Measurements were done in duplicate, and variation was less than 10%. One representative experiment is shown.
NADPH Binding and Kd Determination
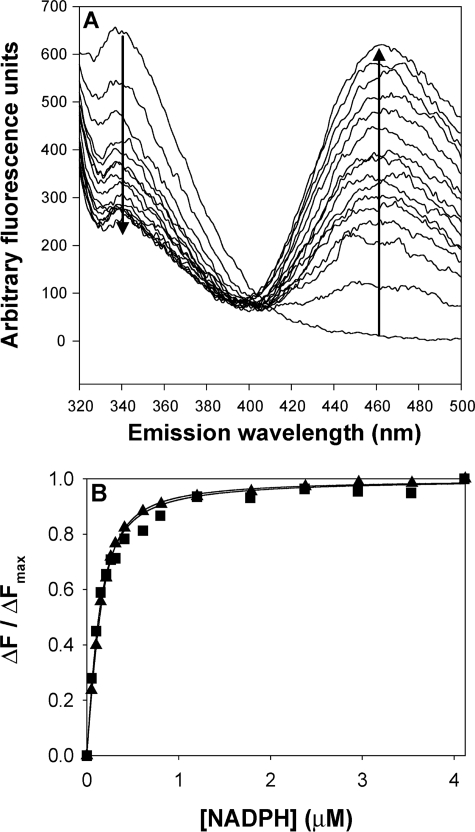
We investigated whether the P133R mutation affects cofactor binding. AKR1D1 contains 5 tryptophan residues and has intrinsic fluorescence when excited at 295 nm. Incremental addition of NADPH quenched the fluorescence emission signal at 340 nm and generated an energy transfer band at 460 nm (Fig. 3A). This energy transfer band probably results from the interaction of the nicotinamide ring with Trp89 based on identical experiments with AKR1C2 and structural homology considerations (27). Plots of ΔF/ΔFmax versus [NADPH] were fitted to the Morrison equation to obtain the dissociation constant (Kd) of NADPH. Wild type AKR1D1 displayed a Kd of 43 ± 16 nm, whereas the P133R mutant was found to bind NADPH with slightly less affinity (Kd = 69 ± 5 nm) (Fig. 3B). However, our enzyme assays contained saturating concentrations of cofactor, and therefore these small differences in Kd are unlikely to account for the large decrease in catalytic efficiency. Interestingly, in the absence of cofactor, AKR1D1-P133R had greater fluorescence emission (20,500 arbitrary fluorescence units/μg of enzyme) at its λmax (343 nm) than wild type (4,700 arbitrary fluorescence units/μg) at its λmax (337 nm).
FIGURE 3.
Titration of the fluorescence emission spectra of AKR1D1 with increasing [NADPH]. A, emission spectrum of recombinant AKR1D1 excited at 295 nm following the addition of increasing concentrations of NADPH from 0 to 4 μm. A decrease in emission is seen at 340 nm, and an increase in emission is seen at 460 nm. B, plot of ΔF/ΔFmax versus [NADPH], wild type (■), and P133R (▴).
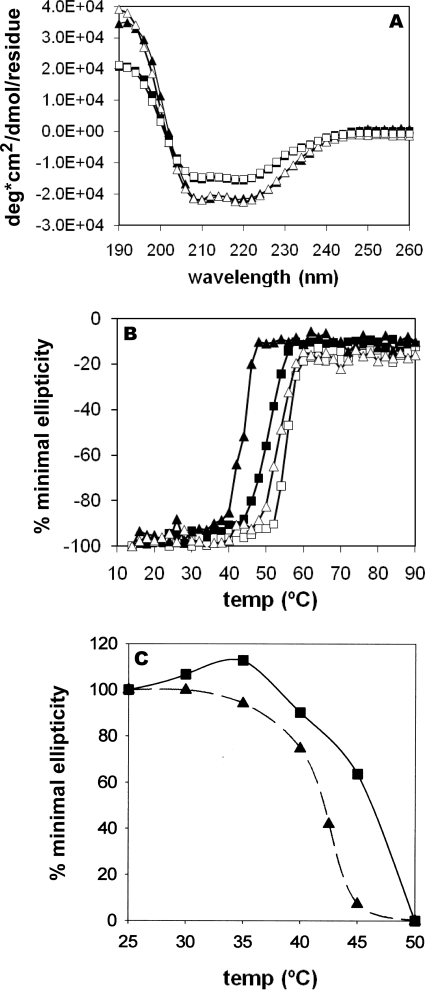
Comparison of Overall Structural and Thermal Stability
CD spectra were recorded for wild type and the AKR1D1-P133R mutant, in the presence and absence of NADPH (Fig. 4A) and showed deflections characteristic of a protein formed of both β-strands and α-helices. Interestingly, the spectra of wild type and AKR1D1-P133R did not superimpose, reflecting some differences in β-strand and α-helical content. To examine the differences between the two proteins further, we performed CD melt curves. AKR1D1-P133R had a Tm of 41 °C, which was lower than wild type AKR1D1 with a Tm of 48.5 °C (Fig. 4B). However, in the presence of cofactor, the differences in Tm were attenuated. Activity measurements of wild type and AKR1D1-P133R following heating at incremental temperatures showed a 50% decrease in enzyme activity at 46.5 and 42 °C, respectively (Fig. 4C). At 46.5 °C, less than 5–10% of the activity of the P133R mutant remained, whereas >60% of the activity remained in wild type enzyme. These temperatures corresponded with the Tm determined in the CD melt experiment without cofactor.
FIGURE 4.
Comparison of the stability of wild type and P133R mutant AKR1D1 using CD spectroscopy and thermal denaturation. A, CD spectra determined for WT AKR1D1 with (□) and without (■) NADPH and AKR1D1-P133R with (Δ) and without (▴) NADPH from 260 to 190 nm. B, melt curve determined for WT AKR1D1 with (□) and without (■) NADPH and AKR1D1-P133R with (Δ) and without (▴) NADPH at 222 nm from 14 to 94 °C. C, activity for WT AKR1D1 (■) and AKR1D1-P133R (▴) heated between 25 and 50 °C for 10 min, followed by a fluorimetric assay with testosterone as substrate. Standard assay conditions were used for measurements. For the heat inactivation experiment shown in C, measurements were performed in duplicate and the mean is given.
AKR1D1 Expression and Protein Stability in HEK293 Cells
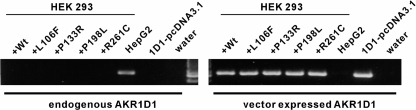
Due to the difficulty to obtain purified natural mutants of AKR1D1, we conducted transient transfection studies. HEK 293 cells do not express endogenous AKR1D1 but express mRNA of wild type and mutant AKR1D1 enzymes to similar degrees following transfection (Fig. 5). However, protein levels were not uniform. Wild type AKR1D1 showed the highest amount of expressed protein, followed by AKR1D1-P133R, whereas low levels of expression were observed for all other mutants and were close to the detection limit of the polyclonal antibody. AKR1D1 protein bands were semiquantified by use of densitometry and compared with a standard curve obtained with recombinant AKR1D1. Wild type AKR1D1 was present at about 0.1% of soluble protein in the cell lysate. The relative expression levels of the wild type and mutant AKR1D1 were 1 (WT):0.33 (P133R):0.034 (G223E):0.027 (P198L):0.004 (L106F and R261C).
FIGURE 5.
Detection of AKR1D1 and AKR1D1 mutant mRNA following transient transfection in HEK 293 cells. HEK 293 cells were transfected with wild type (+Wt) and mutant AKR1D1 (+L106F, +P133R, +P198L, and +R261C), and expression was monitored with primer pairs specifically amplifying either endogenous or vector-expressed (exogenous) AKR1D1. The liver cell line HepG2 expressed AKR1D1 endogenously and served as a positive control for this primer pair. 1D1-pcDNA3.1 is the purified plasmid of the wild type construct transfected into HEK 293 cells and serves as positive control for detection of the exogenous AKR1D1.
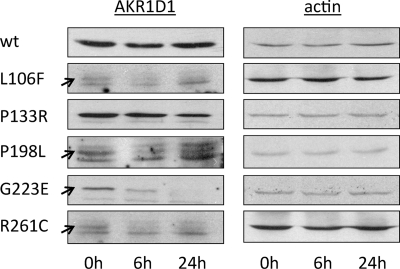
To investigate whether the low expression levels of mutant AKR1D1 were caused by increased degradation rates, we monitored wild type and mutant AKR1D1 protein levels over time following cycloheximide treatment (Fig. 6). Within 24 h after cycloheximide administration, wild type AKR1D1 levels did not decrease significantly, pointing to a fairly stable protein with a low degradation rate. AKR1D1-P133R and AKR1D1-P198L showed a mild reduction in expression level. AKR1D1-G223E decreased significantly within the first 6 h and was no longer detectable 24 h following cycloheximide treatment. AKR1D1-L106F and AKR1D1-R261C were expressed very poorly and degraded within 6 h after cycloheximide treatment. Thus, disease-related mutants of AKR1D1 were associated with poor protein expression and/or stability when expressed in mammalian cells.
FIGURE 6.
Protein expression and stability of AKR1D1 and its natural mutants in transfected HEK 293 cells. Cells were collected at 0, 6, and 24 h following treatment with cycloheximide, and AKR1D1 enzymes were detected by Western blot with a polyclonal anti-AKR1D1 antibody. Blots were stripped and reprobed with antibodies against β-actin to confirm equal loading within an individual experiment. Variations in signal intensity across different constructs are due to differences in exposure time. The arrows point to the signals corresponding to the mutants L106F, P198L, and R261C, that expressed at such low levels that background bands appeared due to increased exposure time.
5β-Reduction of Testosterone by Wild Type and Mutant AKR1D1 in HEK 293 Cells
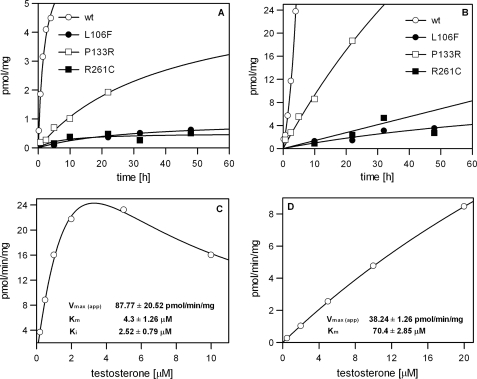
Cells transiently transfected with wild type and mutant AKR1D1 were also assayed for 5β-reductase activity using testosterone as substrate. Three of the five mutants displayed detectable but low 5β-reductase activity when expressed in HEK 293 cells. The observed activities in the presence of 0.5 and 5 μm testosterone in relation to the wild type enzyme are shown in Fig. 7. AKR1D1-P133R activity was significantly reduced compared with wild type enzyme, whereas 5β-reductase activity of the AKR1D1-R261C and AKR1D1-L106F mutants was at the limit of detection of the assay and barely observable in incubation times shorter than 24 h. We did not observe AKR1D1-P198L- or AKR1D1-G223E-mediated 5β-reduction even when incubation times were extended to 60 h (data not shown). Although HEK 293 cells did not display any 5β-reduction of testosterone on their own, testosterone metabolism in incubation times longer than 24 h led to accumulation of downstream products, including 5α-dihydrotestosterone, 3α-androstanediol, Δ4-androstene-3,17-dione, and 5α-androstanedione, which severely hampered quantification of residual 5β-reduction associated with the AKR1D1-R261C and AKR1D1-L106F mutants. Therefore, kinetic parameters were only estimated for wild type AKR1D1 (Fig. 7C) and AKR1D1-P133R (Fig. 7D). Measurement of enzyme activity in the HEK 293 cells revealed many of the same characteristics as observed for the purified enzymes. HEK 293-expressed wild type AKR1D1 exhibited a Km of about 4 μm with testosterone but displayed significant substrate inhibition with a Ki value in the low micromolar range. In contrast, AKR1D1-P133R did not show any substrate inhibition and displayed non-saturable kinetics with a more than 10-fold increase in Km compared with the wild type enzyme.
FIGURE 7.
Detection of 5β-reductase activity of AKR1D1 and its natural mutants in transfected HEK 293 cells. Production of 5β-reduced products from 0.5 μm (A) or 5.0 μm (B) testosterone was followed for up to 48 h in cells expressing either wild type or mutant AKR1D1 or in cells that had been transfected with empty vector (negative control). 5β-Reductase activity for AKR1D1-L106F and AKR1D1-R261C was clearly detectable but close to the detection limit. Cells expressing vector control or AKR1D1-P198L did not exhibit measurable 5β-reduction activity (<0.1 pmol/mg at 60 h of incubation; data not shown). For wild type (C) and AKR1D1-P133R (D), estimates of Km and Vmax were obtained in the transfected cells using testosterone as substrate. All values are normalized to total amount of protein. For A and B, each experiment was performed twice and gave similar results; one representative experiment is shown. For C and D, each point was measured in triplicate with S.E. values less than 10%.
DISCUSSION
Characteristics of Observed AKR1D1 Mutations
Major characteristics of 5β-reductase (AKR1D1) deficiency are an accumulation of the bile acid intermediates (3-oxo-Δ4 sterols), occurrence of 5α-reduced side products (allo-bile acids), and lack of downstream end products (functional bile acids, such as cholic and deoxycholic acid) (7). However, besides defects in AKR1D1 itself, secondary causes that disrupt liver function and bile acid metabolism cannot be ruled out in the presentation of this phenotype (9, 11, 12, 28). In seven patients with 5β-reductase deficiency symptoms, mutations that alter the AKR1D1 amino acid sequence have been identified. Two mutations severely truncate the protein (10, 15), and five mutations result in an amino acid substitution (L106F, P133R, P198L, G223E, and R261C) (8, 10, 15). Although the truncated proteins are considered too short to remain functionally active (29), it is less clear whether the observed point mutations could disrupt 5β-reductase activity because none of these amino acids directly contributes to cofactor binding, substrate binding, or catalysis. It is noteworthy, that among 24 annotated full-length 5β-reductase sequences from various vertebrate species, including nine non-mammalian and five fish sequences, that share about 30% overall identity, all five affected amino acids are 100% conserved. This indicates that the observed mutations may affect overall structure and stability of the enzyme, especially because most substitutions lead to a drastic alteration of physico-chemical properties of the amino acid side chain.
Herein, we show that the introduction of the five disease-associated mutations into AKR1D1 resulted in effects on steady state kinetic parameters and/or levels of expression and protein stability. When these effects are combined, they will strongly decrease physiological 5β-reductase activity, consistent with the observed bile acid metabolic profile seen in affected patients.
Properties of the AKR1D1-P133R Mutant
Only one of the five point mutants, P133R, was purified to homogeneity from E. coli and had detectable activity. The L106F, P198L, and R261C mutants gave very low yields of homogenous protein and/or displayed no detectable activity and accumulated in inclusion bodies, suggesting that in prokaryotic systems, these enzymes are unstable. As a result, the remaining mutants were characterized by mammalian cell expression.
Using the purified AKR1D1-P133R, the mutant was found to have depressed kcat and Km values with bile acid precursors. From these changes in kinetic parameters, it is predicted that bile acid precursors (e.g. Δ4-3-oxo bile acids) would accumulate and/or be directed to form hepatotoxic allo-bile acids, which is the clinical presentation observed. This change is unexpected in the P133R mutant because, based on structures of AKRs in the Protein Data Bank, this residue is not located in a functional region of the protein. Our studies showed an increase in thermal lability, as judged by CD melt curves and thermal denaturation. The attenuation of thermal lability by low micromolar concentrations of NADPH also suggests that this is not the reason for lower enzyme activity because these concentrations of NADPH are likely to be present in the cell and keep the enzyme in its NADPH-bound stable form. Instead, the enzyme behaves as if an uncompetitive inhibitor is present. It is noteworthy that the P133R mutant abolishes the substrate inhibition observed with testosterone, suggesting that this change prevents steroid substrate binding in the unproductive binding mode. A ribbon diagram shows the position of this residue in the structure (Fig. 1A), and its role in the non-productive binding of testosterone responsible for substrate inhibition in wild-type enzyme is shown as a perspective diagram in Fig. 1B. The loss of substrate inhibition observed in this mutant could either be due to direct movement of Tyr132 so that it is unable to hydrogen-bond to testosterone as previously observed (14) or due to a change in the tertiary structure of the enzyme. CD spectroscopy scans of wild type and P133R showed apparent differences in overall β-strand and α-helical content, whereas the P133R mutant red-shifted the tryptophan emission from 337 to 343 nm. This bathochromic shift would represent the tryptophans becoming more solvent-accessible (21) and would also be reflected in a greater emission (as observed).
L106F, P198L, G223E, and R261C Mutants
The remaining mutants were transiently expressed in HEK 293 cells for characterization. Transcript levels of wild type AKR1D1 and all five mutants were similarly expressed when measured by reverse transcription-PCR. Wild type AKR1D1 and P133R were expressed well and showed kinetic properties similarly to the homogeneous recombinant enzymes, suggesting that their properties are not affected by this expression system. By contrast, two mutants, L106F and R261C, were expressed more than 100 times lower than wild type AKR1D1 but showed 5β-reductase activity at the detection limit of the assay indicating that 5β-reductase deficiency syndromes here may not be caused by defects in catalytic properties but by reduced expression of amounts of active protein. The P198L and G223E mutants may decrease physiological 5β-reductase activity due to a combination of effects on enzyme activity as well as expression levels. These mutants were more highly expressed than the L106F and R261C mutants but displayed no detectable 5β-reductase activity.
Relationship of Genotype to Phenotype
It is noteworthy that a single intact copy of the AKR1D1 gene may be sufficient to ensure proper downstream metabolism from the 3-oxo-Δ4 bile acid intermediates and prevent the development of bile acid deficiency symptoms. In all seven cases of a genetic 5β-reductase defect, heterozygous parents or siblings of the patients are healthy and had no reported liver defects. In one case, the serum of a patient's heterozygous mother contained significantly increased levels of the 3-oxo-Δ4 bile acid precursors (15). However, no liver dysfunction was observed. Furthermore, the patient heterozygous for the G223E mutation remained healthy without treatment after initial treatment with ursodeoxycholate was discontinued (15). The compound heterozygous twins reported by Gonzales et al. (8) carried mutations that still retained a significantly active (P133R) mutant and a marginally active (R261C) mutant that together may have contributed to milder symptoms that were successfully treated by cholic acid supplementation. By contrast, two of three reported cases with homozygous mutations in AKR1D1 suffered from severe complications that eventually necessitated liver transplantations (10).
Adaptation to Bile Acid Deficiency
The patient with the homozygous mutation in AKR1D1, P198L, responded well to bile acid supplementation treatment and remained healthy under treatment. Surprisingly, a later study by Palermo et al. (30) on this patient revealed that despite discontinued bile acid supplementation, the girl was healthy. Furthermore, despite strongly reduced levels of 5β-reduced steroids in serum the patient did not exhibit any evidence for a clinical condition associated with dysfunctional steroid metabolism. Although we could not demonstrate residual 5β-reductase activity for the P198L mutant, the presence of low but sufficient enzyme activity to yield low levels of 5β-reduced bile acid precursors may be present in this patient. Furthermore, the presence of a yet unidentified enzyme with 5β-reductase activity in addition to AKR1D1 cannot be ruled out.
The record of this patient also indicates that 5β-reductase activity may be most crucial during early childhood when the infant is initially confronted with establishing the uptake of nutrients from ingested food and development and regulation of bile acid metabolism. In the fetus and in newborns prior to infancy, 3-oxo-Δ4 bile acid intermediate levels are high and similar to the levels observed in the patients (31). However, within the first months of early childhood, these levels dropped significantly, indicating up-regulation of bile acid synthesis and onset of 5β-reductase activity. Inability to achieve this regulation, for example due to deficient 5β-reductase activity, may then result in cholestasis and bile acid deficiency syndromes that require supplemental treatment.
In summary, our data together with the history of affected patients indicate that the observed mutations in AKR1D1 can account for bile acid deficiency and liver damage due to reduced physiological 5β-reductase activity. Bile acid supplementation seems beneficial for these patients to surmount these complications in early life, but adaptive responses in later life indicate that supplementation may not be required.
Supplementary Material
Acknowledgment
We thank Dr. Mo Chen for help in preparing Fig. 1.
This work was supported, in whole or in part, by National Institutes of Health Grants R01-DK47015 and P30 ES013508 (to T. M. P.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Fig. S1.
- TBS
- Tris-buffered saline
- WT
- wild type.
REFERENCES
- 1.Kondo K. H., Kai M. H., Setoguchi Y., Eggertsen G., Sjöblom P., Setoguchi T., Okuda K. I., Björkhem I. (1994) Eur. J. Biochem. 219, 357–363 [DOI] [PubMed] [Google Scholar]
- 2.Deslypere J. P., Young M., Wilson J. D., McPhaul M. J. (1992) Mol. Cell. Endocrinol. 88, 15–22 [DOI] [PubMed] [Google Scholar]
- 3.Wright A. S., Thomas L. N., Douglas R. C., Lazier C. B., Rittmaster R. S. (1996) J. Clin. Invest. 98, 2558–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertilsson G., Heidrich J., Svensson K., Asman M., Jendeberg L., Sydow-Bäckman M., Ohlsson R., Postlind H., Blomquist P., Berkenstam A. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 12208–12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore L. B., Parks D. J., Jones S. A., Bledsoe R. K., Consler T. G., Stimmel J. B., Goodwin B., Liddle C., Blanchard S. G., Willson T. M., Collins J. L., Kliewer S. A. (2000) J. Biol. Chem. 275, 15122–15127 [DOI] [PubMed] [Google Scholar]
- 6.Russell D. W. (2003) Annu. Rev. Biochem. 72, 137–174 [DOI] [PubMed] [Google Scholar]
- 7.Setchell K. D., Suchy F. J., Welsh M. B., Zimmer-Nechemias L., Heubi J., Balistreri W. F. (1988) J. Clin. Invest. 82, 2148–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzales E., Cresteil D., Baussan C., Dabadie A., Gerhardt M. F., Jacquemin E. (2004) J. Hepatol. 40, 716–718 [DOI] [PubMed] [Google Scholar]
- 9.Kimura A., Kondo K. H., Okuda K. I., Higashi S., Suzuki M., Kurosawa T., Tohma M., Inoue T., Nishiyori A., Yoshino M., Kato H., Setoguchi T. (1998) Eur. J. Pediatr 157, 386–390 [DOI] [PubMed] [Google Scholar]
- 10.Lemonde H. A., Custard E. J., Bouquet J., Duran M., Overmars H., Scambler P. J., Clayton P. T. (2003) Gut 52, 1494–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sumazaki R., Nakamura N., Shoda J., Kurosawa T., Tohma M. (1997) Lancet 349, 329. [DOI] [PubMed] [Google Scholar]
- 12.Clayton P. T., Patel E., Lawson A. M., Carruthers R. A., Tanner M. S., Strandvik B., Egestad B., Sjovall J. (1988) Lancet 1, 1283–1284 [DOI] [PubMed] [Google Scholar]
- 13.Ichimiya H., Egestad B., Nazer H., Baginski E. S., Clayton P. T., Sjövall J. (1991) J. Lipid Res. 32, 829–841 [PubMed] [Google Scholar]
- 14.Russell D. W., Setchell K. D. (1992) Biochemistry 31, 4737–4749 [DOI] [PubMed] [Google Scholar]
- 15.Ueki I., Kimura A., Chen H. L., Yorifuji T., Mori J., Itoh S., Maruyama K., Ishige T., Takei H., Nittono H., Kurosawa T., Kage M., Matsuishi T. (2009) J. Gastroenterol. Hepatol. 24, 776–785 [DOI] [PubMed] [Google Scholar]
- 16.Di Costanzo L., Drury J. E., Penning T. M., Christianson D. W. (2008) J. Biol. Chem. 283, 16830–16839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faucher F., Cantin L., Luu-The V., Labrie F., Breton R. (2008) Biochemistry 47, 8261–8270 [DOI] [PubMed] [Google Scholar]
- 18.Penning T. M., Burczynski M. E., Jez J. M., Hung C. F., Lin H. K., Ma H., Moore M., Palackal N., Ratnam K. (2000) Biochem. J. 351, 67–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steckelbroeck S., Jin Y., Gopishetty S., Oyesanmi B., Penning T. M. (2004) J. Biol. Chem. 279, 10784–10795 [DOI] [PubMed] [Google Scholar]
- 20.Jin Y., Stayrook S. E., Albert R. H., Palackal N. T., Penning T. M., Lewis M. (2001) Biochemistry 40, 10161–10168 [DOI] [PubMed] [Google Scholar]
- 21.Komoto J., Yamada T., Watanabe K., Takusagawa F. (2004) Biochemistry 43, 2188–2198 [DOI] [PubMed] [Google Scholar]
- 22.Qiu W., Zhou M., Labrie F., Lin S. X. (2004) Mol. Endocrinol. 18, 1798–1807 [DOI] [PubMed] [Google Scholar]
- 23.Jez J. M., Penning T. M. (1998) Biochemistry 37, 9695–9703 [DOI] [PubMed] [Google Scholar]
- 24.Drury J. E., Di Costanzo L., Penning T. M., Christianson D. W. (2009) J. Biol. Chem. 284, 19786–19790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ratnam K., Ma H., Penning T. M. (1999) Biochemistry 38, 7856–7864 [DOI] [PubMed] [Google Scholar]
- 26.Morrison J. F. (1969) Biochim. Biophys. Acta. 185, 269–286 [DOI] [PubMed] [Google Scholar]
- 27.Jin Y., Penning T. M. (2006) Biochemistry 45, 13054–13063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clayton P. T. (1991) J. Inherit. Metab. Dis. 14, 478–496 [DOI] [PubMed] [Google Scholar]
- 29.Jez J. M., Bennett M. J., Schlegel B. P., Lewis M., Penning T. M. (1997) Biochem. J. 326,, 625–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palermo M., Marazzi M. G., Hughes B. A., Stewart P. M., Clayton P. T., Shackleton C. H. (2008) Steroids 73, 417–423 [DOI] [PubMed] [Google Scholar]
- 31.Inoue T., Kimura A., Aoki K., Tohma M., Kato H. (1997) Arch. Dis. Child Fetal Neonatal Ed. 77, F52–F56 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.