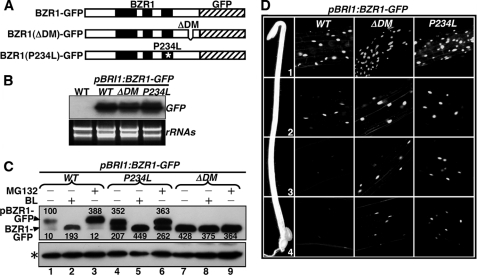
FIGURE 6.
Direct kinase-substrate docking mechanism is responsible for the in vivo phosphorylation of BZR1 by BIN2. A, schematic presentation of three pBRI1:BZR1-GFP fusion genes driven by a BRI1 promoter, including the wild-type and two mutated variants carrying the P234L mutation or lacking the BIN2-DM (ΔDM). B, RNA blot analysis with a GFP probe showing similar levels of transgene expression in three representative pBRI1:BZR1-GFP transgenic lines expressing one of the three transgenes shown in A. The lower panel shows equal loading of total RNAs by ethidium bromide staining. C, deletion of the BIN2-DM prevents BZR1 phosphorylation and inhibits BZR1 degradation in vivo. 3-Week-old seedlings were treated with 1 μm BL or 10 μm MG132 for 2 h in liquid ½ MS medium, and the amount of BZR1-GFP fusion proteins was analyzed by immunoblotting using an anti-GFP antibody. The resulting signals were digitized and quantified by ImageJ (rsbweb.nih.gov). The numbers shown above and below the protein bands in the top panel are relative abundance (%) of phosphorylated (pBZR1-GFP) and nonphosphorylated bands (BZR1-GFP), respectively, to that of the phosphorylated wild-type BZR1-GFP after normalization against the signal intensity of the nonspecific band (indicated by an asterisk). D, deletion of the BIN2-DM enhances the nuclear accumulation of BZR1-GFP in dark-grown hypocotyls. The intensity of GFP fluorescence signal was monitored at different regions of elongating hypocotyls: 1, the region just below the apical hook; 2, the upper middle region; 3, the lower middle region; and 4, the bottom region.