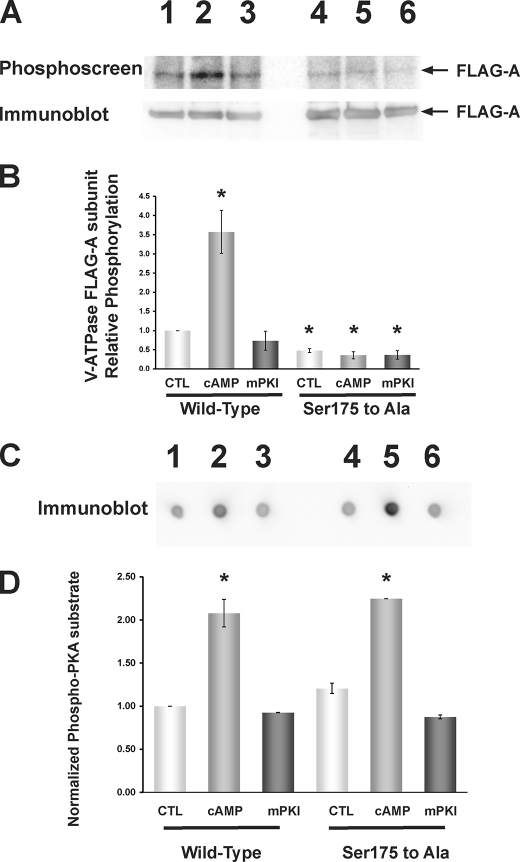
FIGURE 3.
PKA-dependent in vivo phosphorylation of the V-ATPase A subunit in HEK-293T cells occurs at Ser-175. FLAG-tagged WT or S175A mutant A subunit was transfected into HEK-293T cells 1 day before experimentation, and cells were then incubated with [32P]orthophosphate for 2 h under control conditions or in the presence of PKA activator (1 mm 6-MB-cAMP; last 20 min of labeling period) or PKA inhibitor (10 μm mPKI; for entire labeling period). Cell lysis, immunoprecipitation using an anti-FLAG antibody, SDS-PAGE, immunoblotting using an anti-FLAG antibody, and exposure of the same membrane to a phosphoscreen were then performed as described (38). A, typical phosphoscreen image (upper panel) revealing the signal of phosphorylated A subunit in cells expressing FLAG-A-WT subunit (lanes 1–3) or FLAG-A-S175A subunit (lanes 4–6). Lanes 1 and 4 were derived from control-treated cells, lanes 2 and 5 were derived from PKA-stimulated cells, and lanes 3 and 6 were derived from PKA-inhibited cells. The Western blot (lower panel) confirms similar protein expression and loading of the gel for the different conditions. B, quantification of mean (±S.E.) V-ATPase A subunit phosphorylation signal relative to FLAG-A-WT control condition and normalized for protein expression. PKA activator increased FLAG-A-WT phosphorylation to ∼3.5 times that of the control condition, whereas FLAG-A-S175A mutant subunit phosphorylation was reduced across all conditions to ∼0.5 times that of the FLAG-A-WT subunit under the control condition (*, p < 0.05 relative to WT control by analysis of variance; n = 3 replicate experiments). C, dot blots using the PKA phosphorylation substrate-specific antibody of whole cell lysate samples taken from cells transfected and treated under the same conditions as shown in A and B and spotted onto a nitrocellulose filter. D, quantification of mean (±S.E.) PKA-phosphorylated substrate signal relative to that FLAG-A-WT-transfected cell lysates under the control condition and normalized to β-actin blot signal re-probed on the same dot blot (not shown). PKA activator 6-MB-cAMP increased the PKA-phosphorylated substrate signal to 2–2.5 times that of control (*, p < 0.05, relative to FLAG-A-WT control; unpaired t tests), whereas mPKI had no significant effect. As a further control, we measured the levels of [32P]orthophosphate protein labeling in cellular lysates. We did not observe any statistically significant difference across conditions, independently of the A-subunit mutant expressed and pharmacologic treatments (n = 3 per condition; data not shown).