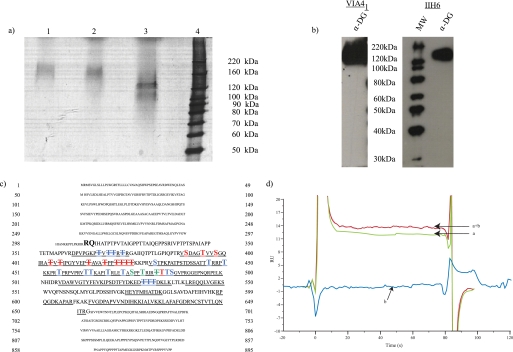
FIGURE 1.
Purified, functionally glycosylated α-DG from rabbit skeletal muscle. a, silver staining following SDS-PAGE of purified α-DG (lane 1). Lanes 2 and 3 represent mock or glycoside (N-glycosidase F, sialidase, endo-O-glycosidase, β(1–4)-galactosidase, and β-N-acetylglucosaminidase)-treated α-DG. b, Western blot analysis following SDS-PAGE of purified α-DG with the glycan-dependent anti-α-DG monoclonal VIA41 and IIH6, which specifically recognizes fully glycosylated, functionally active α-DG. c, protein sequence derived from the dystroglycan gene with the capitalized boldface sequence representing the predicted mature α-DG protein. Peptides assigned by tandem mass spectrometry are underlined. Sites detected to be modified by GalNAc are highlighted in blue; sites of O-mannosylation are highlighted with red; residues observed to be modified by both GalNAc and mannose are green. Sites of potential modification are highlighted similarly and distinguished by striking through the modified residue. d, untreated α-DG (a), α-DG treated with β-galactosidase and sialidase (a + b), or glycosidases alone (b, enzymes without α-DG present) binding to immobilized laminin-1 as measured by surface plasmon resonance.