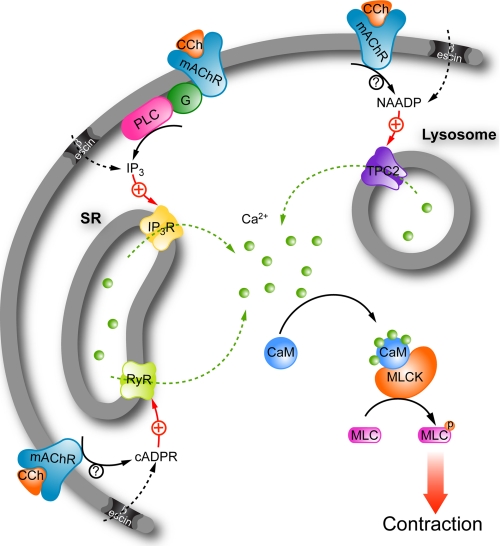
FIGURE 7.
Schematic diagram of receptor-mediated Ca2+-signaling pathways in mouse detrusor muscle. CCh activation of muscarinic acetylcholine receptors (mAChRs) stimulates contraction of detrusor muscle by evoking Ca2+ release from both SR and acidic (lysosomal) stores. Ca2+ binds to calmodulin (CaM), which then activates myosin-light chain kinase (MLCK), which phosphorylates myosin light chains (MLCs), promoting contraction. The three principal Ca2+-mobilizing messengers, IP3, cADPR, and NAADP may all stimulate contractile responses in β-escin permeabilized myocytes, with IP3 and cADPR mobilizing Ca2+ from the SR, by activating IP3 receptors and RyRs, respectively, and NAADP from acidic stores by activating TPC2 channels. IP3 production is stimulated by mAChR via G proteins (G) coupling to phospholipase C (PLC), whereas cADPR and NAADP synthesis may be controlled by ADP-ribosyl cyclases such as CD38. mAChRs require the expression of TPC2 and action of NAADP for coupling to Ca2+ release from acidic stores (lysosomes). Greater mAChR stimulation was required to effect coupling to Ca2+ release from acidic stores when compared with the SR, raising the possibility of weaker coupling to NAADP synthesis or selective coupling of a lower affinity mAChR subtype to NAADP synthases.