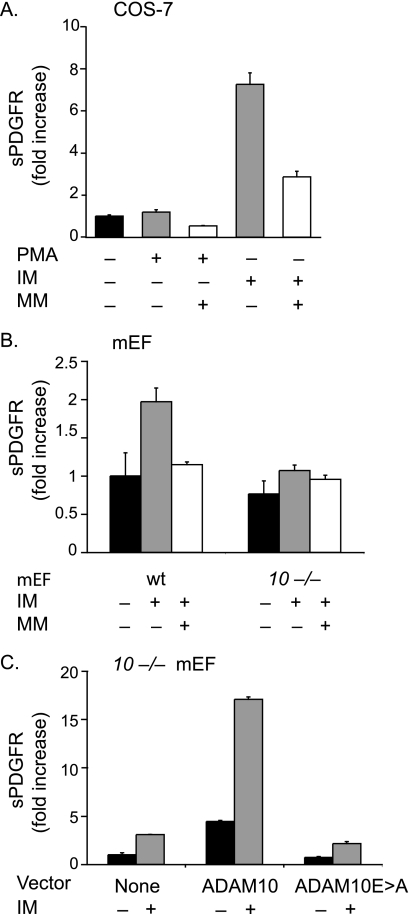
FIGURE 1.
Ectodomain shedding of PDGFRβ by ADAM10. A, shedding of PDGFRβ-AP transfected into COS-7 cells following stimulation with PMA (25 ng/ml) or ionomycin (2.5 μm) in the presence or absence of the hydroxamate-type metalloproteinase inhibitor marimastat (4 μm). Shedding of PDGFRβ-AP is stimulated by IM, but not by PMA, and the constitutive and IM-dependent shedding can be partially reduced by MM (n = 4 ± S.D.). The constitutive PDGFRβ-AP shedding was set to 1 and used as a reference point to determine the fold increase shedding of all samples. B, shedding of PDGFRβ-AP transfected into wild type (wt) or Adam10−/− (10−/−) mEFs (n = 3 ± S.D.) following stimulation with 2.5 μm IM in the presence or absence of 4 μm MM shows that the IM-stimulated component of PDGFRβ-AP shedding is strongly decreased in the absence of ADAM10. The constitutive PDGFRβ-AP shedding in wild type mEFs was set to 1 and used as a reference point to determine the relative fold increase shedding of all samples. C, shedding of PDGFRβ-AP from Adam10−/− mEFs co-transfected with or without ADAM10 or the ADAM10Glu→Ala mutant, which carries an inactivating Glu→Ala point mutation in its catalytic site, in the presence or absence of 2.5 μm IM (n = 4 ± S.D.). Please note that the differences in fold increase shedding in IM-treated samples are due to different responses of the various cell lines to these stimuli. The low constitutive shedding in Adam10−/− cells was set to 1 in C, hence the relatively high numbers for the fold increase in this panel.