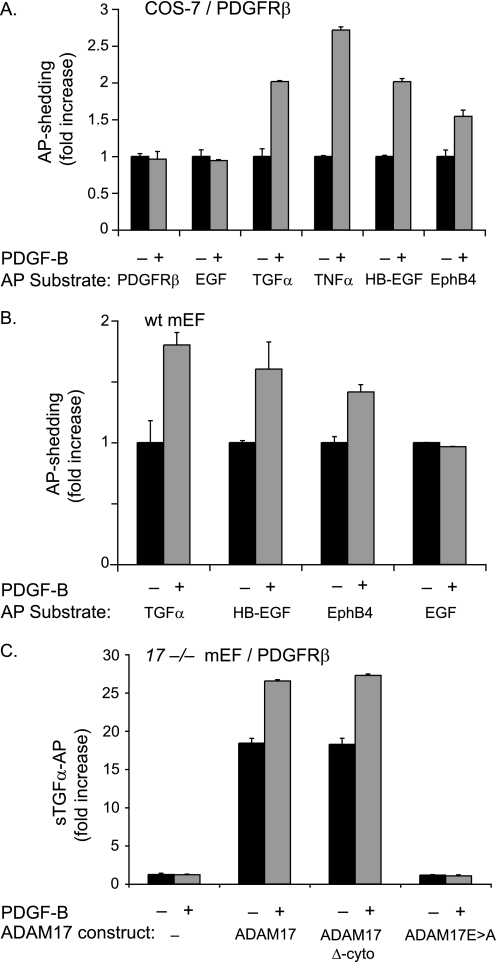
FIGURE 3.
PDGF-B binding to its receptor PDGFRβ does not activate shedding of PDGFRβ-AP or of the ADAM10 substrate EGF but causes shedding of substrates of ADAM17. A, COS-7 cells were co-transfected with full-length PDGFRβ and the following alkaline phosphatase-tagged membrane-anchored substrate proteins: PDGFRβ, EGF, TGFα, TNFα, HB-EGF, and EphB4. The day following transfection, cells were starved by incubation in serum-free medium (Opti-MEM) for 6 h and then incubated for 30 min in the presence or absence of PDGF-B (50 ng/ml). PDGF-B did not stimulate the shedding of the ADAM10 substrates PDGFRβ or EGF but stimulated shedding of all ADAM17 substrates tested here (TGFα, TNFα, HB-EGF, and EphB4) (n = 6 ± S.D.). B, wild type (wt) mEFs were transfected with alkaline phosphatase-tagged TGFα, HB-EGF, EphB4, or EGF and treated with or without 50 ng/ml PDGF-B for 30 min (as in A). PDGF-B stimulation of wild type mEFs, which express the PDGFRβ endogenously, activated shedding of the ADAM17 substrates TGFα, HB-EGF, and EphB4 but not of the ADAM10 substrate EGF (n = 5 ± S.D.). The constitutive shedding (for 30 min) for each AP-tagged substrate in A and B was set to 1 to provide a reference point for the shedding in the presence of PDGF-B (30 min). C, Adam17−/− mEFs (17−/− mEF) transfected with full-length PDGFRβ and TGFα either alone or with wild type ADAM17, ADAM17 lacking its cytoplasmic domain (ADAM17 Δ-cyto), or the catalytically inactive ADAM17Glu→Ala. Cells were stimulated with 50 ng/ml PDGF-B for 30 min or left untreated (n = 6 ± S.D.). The stimulation of TGFα shedding by PDGF-B/PDGFRβ requires a functional catalytic site of ADAM17 but not its cytoplasmic domain. The low level of constitutive shedding of TGFα in untreated Adam17−/− mEFs was set to 1 and used as a reference in C.