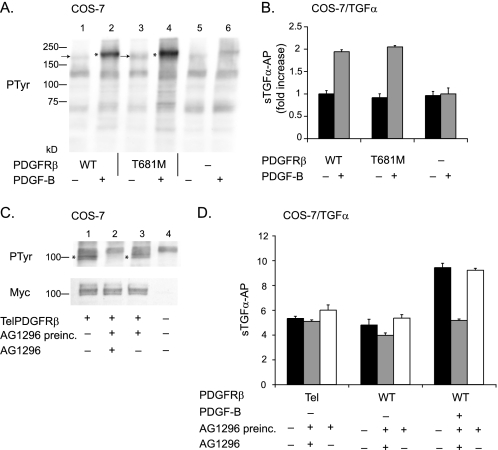
FIGURE 4.
Constitutively active forms of PDGFRβ, one of which is highly phosphorylated, do not stimulate ADAM17. Two constitutively active forms of the PDGFRβ, the gatekeeper mutant (T681M) (A and B), and the TelPDGFRβ (C and D) were tested for their ability to activate ADAM17-dependent shedding of TGFα. A, Western blot of tyrosine phosphorylation (PTyr) of the wild type (WT) receptor and gatekeeper mutant in the presence or absence of PDGF-B, as indicated. Lanes 1–4 show samples of COS-7 cells transfected with wild type PDGFRβ (lanes 1 and 2) or the PDGFRβ T681M (lanes 3 and 4) and left unstimulated (lanes 1 and 3) or stimulated with 50 ng/ml PDGF-B (lanes 2 and 4). Samples from untransfected COS-7 cells that were not treated (lane 5) or treated with 50 ng/ml PDGF-B (lane 6) are shown as controls. The arrows next to lanes 1 and 3 point to the unstimulated wild type PDGFRβ and PDGFRβ T681M mutant, and the asterisks next to lanes 2 and 4 indicate the position of the phosphorylated forms of these receptors after treatment with 50 ng/ml PDGF-B. The phosphorylation of the PDGFRβ T681M mutant in unstimulated cells is only slightly increased compared with the wild type receptor, and the relative increase in phosphorylation upon stimulation with PDGF-B is similar for the wild type and PDGFRβ T681M mutant. B, comparison of TGFα shedding from COS-7 cells transfected with wild type PDGFRβ or PDGFRβ T681M showed no significant difference in constitutive or PDGF-B-stimulated conditions (n = 6 ± S.D.). There was no PDGF-B stimulation of TGFα shedding in COS-7 cells that were not co-transfected with the wild type PDGFRβ. In this graph, the unstimulated shedding of TGFα in cells expressing the wild type receptor was set to 1 and used as a reference to calculate the fold increase in shedding for the other samples. C, Western blot analysis of COS-7 cells transfected with the Myc-tagged TelPDGFRβ probed with an anti-Myc antibody (lower panel) shows comparable expression of this receptor in transfected cells (lanes 1–3) and no expression in untransfected control cells (lane 4). The band detected by the anti-Myc antibodies migrates as a doublet of ∼100 kDa, as described previously for the TelPDGRFβ (20, 21). Tyrosine phosphorylation of the TelPDGFRβ was detected with an anti-phosphotyrosine antibody in samples from transfected but untreated cells (upper panel, lane 1), and this was inhibited following 1 h of preincubation with 20 μm of the PDGFRβ-selective tyrosine kinase inhibitor AG1296 (upper panel, lane 2). When the cells were first incubated with 20 μm AG1296 followed by washing to remove the inhibitor, TelPDGFRβ phosphorylation was restored (top panel, lane 3). D, functional analysis of the ability of the TelPDGFRβ to activate ADAM17 using TGFα shedding assays in COS-7 cells co-transfected with TelPDGFRβ or the wild type PDGFRβ. There was no significant difference in the constitutive shedding of TGFα in COS-7 cells expressing TelPDGFRβ compared with the wild type PDGFRβ in the presence (gray bar) or absence of AG1296 (black bar) or following washout of AG1296 after preincubation for 1 h (white bar). The addition of PDGF-B to COS-7 cells expressing the wild type PDGFRβ and TGFα resulted in a strong increase in TGFα shedding that was blocked by AG1296 but was restored following washout of AG1296. The experiments with the ligand-stimulated wild type PDGFRβ provide a positive control for the ability of AG1296 to block the function of the PDGFRβ and of the reversibility of this inhibition when AG1296 is washed out following preincubation.