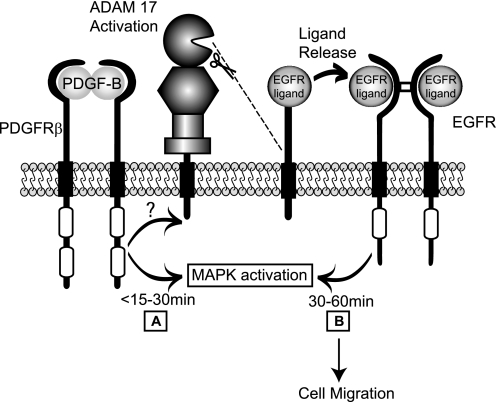
FIGURE 7.
Model for metalloproteinase-dependent cross-talk between the PDGFRβ and the EGFR/ERK1/2 signaling pathway. Based on the results of this study, we propose a model in which binding of PDGF-B to the PDGFRβ causes a biphasic activation of ERK1/2 with an initial response triggered via PDGFRβ-dependent stimulation of intracellular signaling pathways (A) and the second response by activation of ADAM17 (B). This second response results in the processing and release of membrane-anchored EGFR ligands, allowing them to bind to and activate the EGFR. The metalloproteinase-dependent activation of the EGFR is responsible for the extended duration of ERK1/2 phosphorylation between about 30 and 60 min after addition of PDGF-B and for ligand-induced cell migration (B).