Abstract
Previous work has implicated the cholinergic system in modulating feeding behavior; however its specific function remains unclear. The present work aimed to characterize potential dissociations between the central cholinergic modulation of the incentive properties of food and food-associated cues, versus consummatory behaviors. Three separate experiments demonstrated that intra-accumbens infusion of muscarinic antagonist scopolamine, 3 hours prior to the testing session, significantly decreased food intake. General motor activity in anticipation of food was not diminished. Experiments also showed that scopolamine did not impair operant responding for a food-associated conditioned reinforcer (CR) nor was d-amphetamine potentiation of CR responding altered by scopolamine pretreatment. This study contributes to the growing evidence that goal-seeking behaviors are mediated by a distinct set of neural processes than those governing food ‘reward.’
Keywords: scopolamine, muscarinic, accumbens, feeding, acetylcholine
INTRODUCTION
Increases in body weight and body mass have become significant problems in society today, particularly in the United States; the over-consumption of high fat and calorically dense foods greatly contributes to the ‘obesity epidemic’ and associated diseases. The rapid rise in obesity rates in a relative short time suggests that environmental factors, such as those relevant to the affective or motivational control of appetite, can overwhelm the controls exerted by energy homeostasis. Thus, a great deal of research examining neural control of feeding behavior has taken place to try to gain a better understanding of the mechanisms underlying “non-homeostatic” feeding. A brain region of particular interest in the control of appetitive motivation and feeding behaviors is the nucleus accumbens (Acb). The combination of afferent projections from affect-related limbic regions, ascending projections from midbrain dopamine systems, and efferent projections to extrapyramidal motor control centers makes the Acb a well-situated brain region to modulate motivated behaviors in a general sense (Mogenson, Jones, & Yim, 1980). A highly influential theory was proposed by Mogenson, et al. who hypothesized that the Acb was an interface between limbic and motor systems, translating motivational signals into behavioral output (Mogenson et al., 1980). This general idea has been upheld by decades of research (Baldo & Kelley, 2007; Berridge, 2004; Kelley, Baldo, Pratt, & Will, 2005; Salamone, Correa, Farrar, & Mingote, 2007; Wise, 2004). Of particular relevance here is that the pharmacological manipulation of distinct Acb-localized neurotransmitter systems has been shown to produce dissociable effects on food intake and measures of food motivation .
It is useful to consider the discrete profiles produced by the manipulation of the dopamine and opioid systems within the Acb. There is much evidence that dopamine receptor blockade or dopamine-depleting lesions markedly reduce anticipatory/preparatory behaviors associated with feeding and instrumental responding for food or food-associated cues, particularly when these tasks are motorically demanding (Blackburn, Phillips, & Fibiger, 1987; Salamone, 1994; Salamone, Arizzi, Sandoval, Cervone, & Aberman, 2002; Salamone et al., 2007; Zhang, Balmadrid, & Kelley, 2003). For example, bilateral intra-cerebral infusions of D1-or D2-antagonists decrease general locomotor behavior and impair food handling and hoarding without altering latency to begin feeding or total consumption of food (Baldo, Sadeghian, Basso, & Kelley, 2002); (Kelley & Stinus, 1985; Salamone, Mahan, & Rogers, 1993). Also, dopamine depletion in the Acb decreases lever pressing and break point in operant tasks and shifts behavior towards low-effort options, but, again, does not alter total consumption of food (Aberman & Salamone, 1999) (Aberman, Ward, & Salamone, 1998; Salamone et al., 2002). Conversely, intra-Acb dopamine activation (i.e. via amphetamine administration) increases operant responding for food and food-associated stimuli, but does not strongly or consistently affect intake and in some studies decreases in intake (Kelley & Delfs, 1991; Kelley, Gauthier, & Lang, 1989; Zhang et al., 2003). In contrast to dopamine manipulations, blockade of Acb μ-opioid receptors reliably decreases food intake, and conversely opioid receptor stimulation in the Acb produces a striking hyperphagia (for review see(Kelley, Baldo, Pratt et al., 2005). It has been shown that stimulation of Acb-localized-opioid receptors, specifically the μ-receptor, in satiated animals induces marked intake of highly palatable food, while μ-receptor antagonism significantly decreases food consumption, with greater dose-sensitivity for palatable foods versus standard chow (Bakshi & Kelley, 1993a; Kelley, Bless, & Swanson, 1996; Pecina & Berridge, 2005; Ragnauth, Moroz, & Bodnar, 2000; Zhang, Gosnell, & Kelley, 1998; Zhang & Kelley, 2000). Mu-receptor stimulation also increases operant responding for food reward in a progressive ratio paradigm (Zhang et al., 2003); however, μ-opioid agonists do not consistently enhance lever pressing for food-associated conditioned reward (Cunningham & Kelley, 1992). These observations have contributed to an emerging hypothesis that the Acb dopamine system is an important modulator of anticipatory/preparatory behaviors, signaling the incentive value of food and ‘energizing’ goal-seeking behavior, while Acb opioid transmission augments the rewarding properties of the food, once encountered and tasted, resulting in subsequent increased motivation to obtain the food (Baldo & Kelley, 2007; Berridge, 2007; Kelley, Baldo, & Pratt, 2005; Salamone et al., 2007). In strong support of this hypothesis is data from taste reactivity experiments that show neither activation nor depletion of dopamine has an effect on orofacial taste reactions to orally delivered liquid sucrose; however, activation of the μ-opioid receptor enhances the number of positive taste reactions to the sweet solution, supporting the idea of an augmentation of the rewarding value of palatable tastants (Pecina & Berridge, 1995). This effect has further been studied by the use of Fos plumes within the Acb (Pecina & Berridge, 2000), demonstrating a rostral-caudal, positive-aversive gradient within the Acb shell (Pecina & Berridge, 2005).
Guided by this framework, the present study was designed to identify the feeding-related motivational process mediated by the cholinergic muscarinic system in the ventral striatum. Acetylcholine is present throughout the striatum, including the Acb, in large, aspiny neurons which make up about 1-2% of the total striatal neuronal population (Kelley, Baldo, & Pratt, 2005; Zhou, Wilson, & Dani, 2002). It has been shown that intra-Acb infusion of general muscarinic antagonist scopolamine strongly decreases food intake up to 24 h, reduces the break point of a food-reinforced progressive ratio schedule, and attenuates the voracious feeding induced by opioid stimulation (Hoebel, Avena, & Rada, 2007; W. E. Pratt & Kelley, 2004, 2005; Will, Pratt, & Kelley, 2006). These effects could be the result of either an altered valuation of taste or reward properties, causing a subsequent disinterest in eating and therefore a decrease in intake, or from a general negative motivational state that would non-specifically disrupt ingestive behavior. However, a complication arises with some of these previous studies; scopolamine induces a marked motor activation that lasts about 30 min post injection (Joyce & Koob, 1981; W. E. Pratt & Kelley, 2004). This could potentially confound the measurement of food intake and operant responding simply due to behavioral competition and/or performance deficits arising from altered activity, attention, and arousal. Recently, our laboratory has shown that the hypophagic effects of intra-Acb scopolamine are long lasting, significantly outlasting the acute hyperactivity induced by the drug (Perry, Baldo, Andrzejewski, & Kelley, 2009). Therefore, to avoid scopolamine’s acute motor-stimulatory effects, testing was conducted 3 h after intra-Acb drug infusions for all experiments presented here.
The aim of the present study was to determine the component of feeding affected by intra-Acb infusions of scopolamine: anticipatory food-seeking behaviors, food consumption, or both. To address these questions, we examined the effects of scopolamine on feeding microstructure, food intake, motor behavior exhibited in anticipation of feeding, operant responding for a food-associated conditioned stimulus, and amphetamine-potentiation of such responding. These experiments were designed to investigate whether scopolamine’s effects would lie primarily in the modulation of anticipatory food-seeking actions, or in the regulation of neural processes relevant to the feeding consummatory act.
GENERAL METHODS
Experimental Subjects
Seventy-seven male Sprague-Dawley rats (Harlan, Madison, WI, USA) were used in these studies. Rats were housed in pairs in clear plastic cages kept in a temperature- and light-controlled vivarium (12 h light/dark cycle, lights on at 07:00 h). Subjects weighed 275-290 g upon arrival in the laboratory and were initially maintained on an ad libitum feeding schedule. Prior to start of experiments, rats were placed on a food-restriction regimen resulting in maintenance at 85% of free-feeding body weight. Subjects were handled daily to minimize stress. All procedures related to experimental manipulations and animal care were performed according to NIH guidelines on the use of animals in research and regulated by the University of Wisconsin-Madison Medical School Animal Care and Use Committee.
Surgery
Rats were anesthetized with either a Ketamine-Xylazine mixture (100/10 mg/kg) administered intraperitoneally or with isoflurane (1.5 – 2.5%) using an inhalant anesthesia system (Summit, Bend, OR) with oxygen being delivered at all times during the procedure (0.9 liters/minute). Standard aseptic procedures were utilized to implant 10-mm indwelling stainless steel guide cannulae (23 gauge) bilaterally 2.5 mm above the Acb (1.3 mm anterior and 1.7 mm lateral to Bregma; 5.3 mm ventral to skull surface). Guide cannulae were affixed to the skull with the use of screws and dental acrylic (Lang, Henry Schein Inc, Melville, NY). Stylets were placed in the cannulae to prevent occlusion. Rats received an IM injection of buprenorphine (0.30 mL of 0.03 mg/ml) for pain and recovered for at least 7 days prior to behavioral testing.
Drugs and Microinfusion
The following drugs were dissolved in sterile 0.9% saline: muscarinic antagonist scopolamine methyl bromide (10 ug/ 0.5 ul/ side: Sigma Aldrich) and the dopamine indirect agonist d-amphetamine (2.5 μg/0.5 μl/ side: Sigma Aldrich). Scopolamine and amphetamine doses were chosen on the basis of data from previous experiments in this laboratory showing clear behavioral effects (Bakshi & Kelley, 1993b; Cunningham & Kelley, 1992; Kelley et al., 1996; Kelley & Delfs, 1991; Will et al., 2006). The scopolamine dose chosen for the current work has been used in our previous studies showing strong suppression of feeding both acutely and at delayed post-injection time points. This dose is also at the higher end of the dose-effect range for central infusions as reported in the literature (Perry et al., 2009; W. Pratt et al., 2007; W. E. Pratt & Kelley, 2004, 2005; Shannon & Peters, 1990; Will, Franzblau, & Kelley, 2003; Will et al., 2006).
Drugs were administered bilaterally through stainless steel injectors (30 gauge) connected via polyethylene tubing (PE-10) to a microdrive pump (Harvard Apparatus, South Natick, MA). Injectors protruded 2.5 mm below the guide cannulas to the final injection site (7.8 mm below skull surface). Rats were gently hand-held during the infusion process that lasted 2 min 33 s. Habituation to drug infusion began with a mock infusion during which injectors were lowered to the bottom of the guide cannulas and the infusion pump was activated (to habituate rats to infusion-related auditory stimuli), but no infusions were made. The next day saline was infused using thirty gauge injectors extending 2.5 mm beyond the end of the guide cannula. The rate of injection was 0.32 μl/min for all drugs, and the total duration of infusion was 93 s. Total volume infused was 0.5 μl. One additional minute was allowed for diffusion of injectate into the tissue. Injectors were removed, stylets were replaced and rats were returned to their home cage. The same procedure was used to administer drug on testing days.
Histology
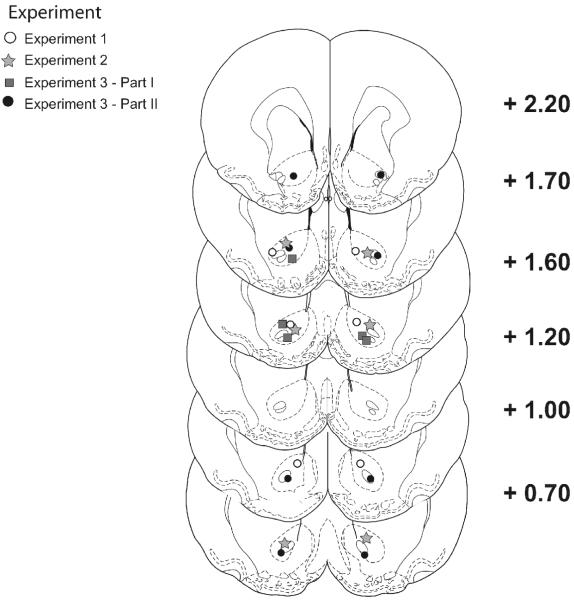
Following behavioral testing, rats were overdosed with sodium pentobarbital and perfused transcardially with saline (200 ml), followed immediately by 500 ml of a 10% buffered formalin solution. The brains were then removed and placed in 10% buffered formalin - 10% sucrose solution overnight. Frozen serial sections (60 μm) were collected through the entire extent of the injection sites, mounted on gelatinized slides, stained with Cresyl violet and cover slipped. Cannulae placements were then assessed with light microscopy by an observer blind to the behavioral results of the rats. Line drawings of representative acceptable placements are shown in Figure 1. One animal was removed from Experiment 1 and 2 were removed from Experiment 2 due to inaccurate placements.
Figure 1.
Line drawings for coronal rat brain sections showing representative cannulae placements for representative animals in each experiment. The stereotaxic coordinates shown are in mm anterior of Bregma.
Statistical Analysis
For Experiment 1, a one way repeated measures ANOVA was used to analyze food intake and latency to the initial feeding bout; ambulations, rears, bout duration and number of bouts initiated were assessed with a two way repeated measures ANOVA (factors: drug and time bin) with Bonferroni-corrected t-tests used for post hoc analysis dependent upon significant effects in the ANOVA (the alpha value was set at 0.05). Food intake data for Experiment 2 was analyzed using a t-test while screen approaches, ambulations and rears were analyzed with of a between-subjects two way ANOVA (factors: group and drug) with the Bonferroni-corrected t-test, as indicated. In Experiment 3 a t-test was used to compare food intake and lever presses between the saline- and scopolamine-treated groups. For the scopolamine-amphetamine interaction on CR a between subjects two way ANOVA (factors: scopolamine pretreatment and d-amphetamine treatment) with Bonferroni-corrected t-tests were used to analyze lever pressing.
Experiment 1: Tests of feeding microstructure and general motor activity
Methods
Behavioral testing was carried out in clear polycarbonate cages (9.5 in. width × 17 in. length × 8 in. height) with wire grid floors. A pre-weighed quantity of food (45 mg sucrose pellets, BioServ, Frenchtown, NJ) was placed in a dish secured to the wire grid floor, and water was available from an overhead bottle. A sheet of paper was placed underneath each testing cage to collect food spillage.
Eight rats were utilized in this experiment to examine feeding behavior 3 h following scopolamine treatment. On testing day, rats were moved from the vivarium and placed in a separate room 3 h prior to test session, given an infusion of saline or scopolamine (10 ug/ 0.5 ul), and returned to their home cage. Each rat received both treatments, saline and scopolamine, counterbalanced across rats, in a within-subject design. At the start of the session, rats were placed into testing cages and behavior was monitored and recorded by an experimenter blind to treatment. The behaviors recorded were: ambulation, defined as whole body movements across the center of the cage; rears, feeding, and drinking. For feeding and drinking, total number of bouts, and duration of each bout, were recorded, whereas for locomotion and rearing only the number of events was recorded. For ingestive behaviors, the beginning of each bout was counted from the time of contact with the food pellet or water spout, and the bout was considered to be ended when sustained eating or drinking was interrupted by a different behavior (for example, ambulation or rearing), with no contact with the food or water for 3 s.These parameters were recorded using a keypad interfaced to a PC computer using ButtonBox5 (Behavioral Research Solutions). Data for the 30 min test session were divided into six 5 min time bins. At the conclusion of the session, uneaten food, food spillage, and remaining water were weighed and recorded.
To measure activity in the absence of food, rats were tested in clear polycarbonate cages (9.5 in. width × 17 in. length × 8 in. height) positioned within a rectangular frame of photobeam sensors interfaced to a personal computer. Two arrays of infrared photobeams (bottom row: 4 beams along long axis, 3.5 inches apart, 2 inches above the floor; top row: 8 beams along short axis, 1 inch apart, 6.5 inches above the floor) were placed on the cage sides to measure rats’ locomotor activity (Photobeam Activity System, San Diego Instruments). These cages had never been associated with food delivery and therefore represented a neutral environment to record locomotor behavior. After five 45 min habituation sessions, rats received saline or scopolamine (10 ug/ 0.5 ul) 3 h prior to the 45 min test session. Treatments were counterbalanced across rats, in a within subject design. Beam breaks were recorded as ambulations or rears.
Results
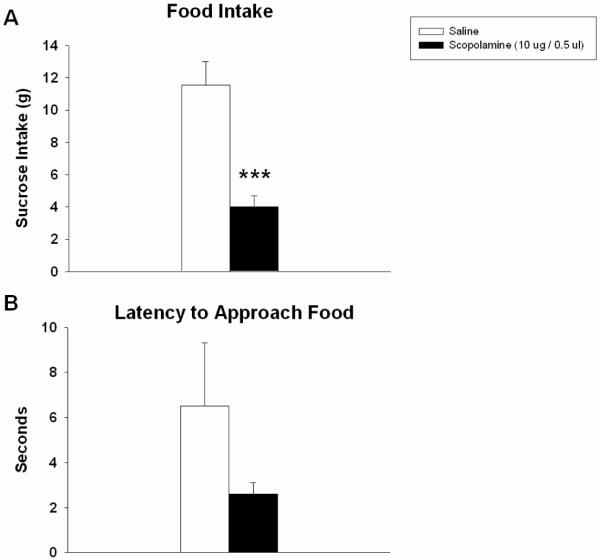
Analysis of food intake 3 h following scopolamine infusion showed a significant decrease in sucrose consumption with drug treatment (F(1,6) = 30.387, p < 0.001; Fig 2a). Differences in the latency to approach the food were not statistically significant; nevertheless, scopolamine-treated rats exhibited a non-significant trend towards approaching the food earlier in the session compared to controls (F(1,6) = 1.689, n.s.; Fig 2b).
Figure 2.
Intra-Acb scopolamine (10 μg/ 0.5 ul) significantly decreased sucrose pellet intake 3 h post infusion (A), but did not alter the latency to approach food compared to saline controls (B). Error bars depict one SEM. (*** p ≤ 0.001, significantly different from saline group)
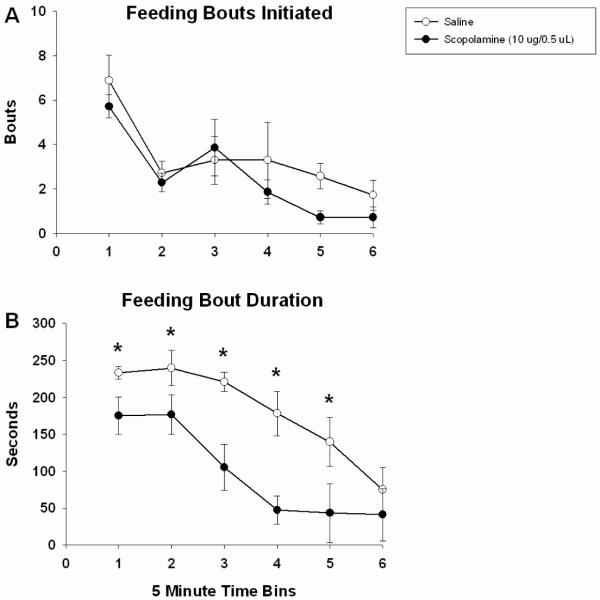
Further examination of food intake revealed differences in the structure of feeding bouts with drug administration. The number of bouts initiated by scopolamine-treated rats did not differ from saline-treated rats (F(1,6) = 1.365, n.s.; Fig 3a); the number of bouts decreased equivalently in both groups with time over the course of the testing session (main effect of time; F(5,30) = 18.290, p < 0.01; Fig 3a). There was no drug x time interaction (F(5,30) = 0.826, n.s.; Fig 3a). In contrast, 3 h scopolamine pretreatment significantly decreased time spent feeding relative to saline treatment (F(1,6) = 56.534, p < 0.001; Fig 3b). In both groups, bout duration significantly decreased over the testing session (main effect of time, F(5,30) = 9.950, p < 0.001; Fig 3b). There was no drug x time interaction (F(5,30) = 1.712, p = 0.162), but based on our a priori hypothesis and multiple, strong main effects, a Bonferroni-corrected t-test was used to compare the average means at each time bin. There were significant differences between saline and scopolamine-treated animals beginning with the first time-bin; it took until the final time-bin of the session for saline-treated rats to exhibit equivalently low levels of time spent feeding as did scopolamine-treated rats (p < 0.05; Fig 3b).
Figure 3.
The number of feeing bouts initiated during the 30 min test session was unaffected 3 h following intra-Acb infusion of scopolamine (10 μg/ 0.5 ul) (A). The length of time spent feeding following drug treatment was significantly decreased compared to saline controls (B). Error bars depict one SEM. (* p ≤ 0.05, significantly different from saline)
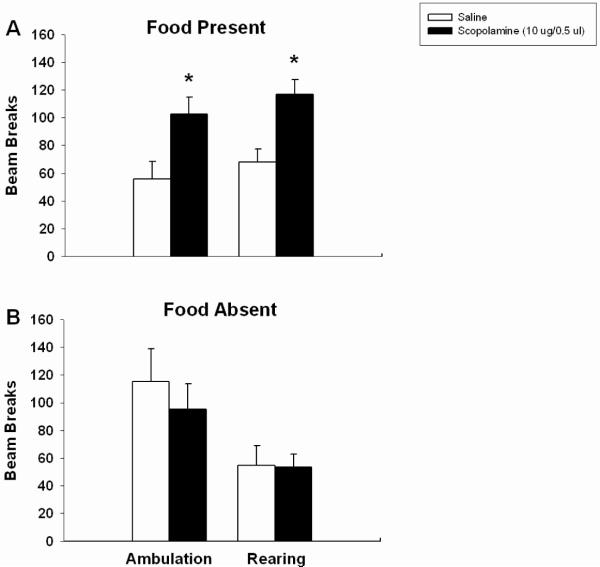
Examination of general motor activity in the presence of food during the feeding microstructure test showed both ambulations and rears were significantly increased 3 h after intra-Acb scopolamine (F(1,6) = 9.211, p < 0.03; F(1,6) = 25.674, p < 0.003, respectively; Fig 4a). However, when rats were placed in a neutral environment (i.e. separate testing cages without food present), the 3 h scopolamine-treated group did not differ from controls with regard to ambulation or rearing behavior (F(1,5) = 0.334, n.s.; F(1,5) = 0.006, n.s., respectively, Fig 4b). These results suggest that the hyperactivity induced in the presence of food 3 h following intra-Acb scopolamine is not simply a pure drug effect, but rather stems from an interaction between drug, testing environment, and proximity of food.
Figure 4.
Effects of scopolamine on ambulation and rearing in the presence and absence of food. Scopolamine-treated rats ambulated and reared significantly more times than saline controls (A). No increase in motor activity was observed in an environment without food association (B). Error bars depict one SEM. (* p ≤ 0.05, significantly different from saline)
Experiment 1: Discussion
Analysis of the feeding microstructure of rats in the presence and absence of food at the 3 h delayed time point yielded several important results. First, as previously shown, rats receiving a scopolamine treatment 3 h prior to testing ate significantly less food than saline controls. Interestingly, the scopolamine-treated rats tested in the presence of food were hyperactive even 3 h after drug infusion. However, when tested in a neutral environment in the absence of food, this activity change was not evident. These effects could suggest that the hyperactivity in the presence of food was due to a drug/environment interaction (e.g., the presence of an appetitive goal ‘releasing’ heightened activity in scopolamine-treated rats), or simply a consequence of altered competition between time spent eating and time spent locomoting. Experiment 2 was designed, in part, to discriminate between these possibilities. In a general sense, the lack of motor deficits would tend to argue against a negative motivational state as the explanation for the feeding decrease. Furthermore, the fact that scopolamine-treated rats approached the food source with equal (or slightly quicker) latency than controls, and initiated a similar number of feeding bouts as controls, would also support the absence of a global motivational deficit. Rather, the marked decrease in total feeding duration (with no change in bouts initiated) would imply a change in sensory or reward processes associated with actual commerce with the food.
Experiment 2: Tests to distinguish drug effects on anticipatory and consummatory behaviors
Methods
Thirty rats were used in this experiment to separately examine changes in anticipatory/preparatory versus consummatory behaviors following a 3 h scopolamine pretreatment. All behavioral testing began one week following surgery, in a room separate from the animal colony containing automated activity/feeding monitors (Med Associates, St. Albans, VT). The sides and top of the locomotion/feeding cage were made of clear Plexiglas with a wire floor. Food intake monitors (Med Associates) were mounted on the sides of the cage and were able to measure food weight with an accuracy of 0.1 g. Two arrays of infrared photobeams were mounted on the front and back of the cage to measure locomotor activity of the rats (bottom row: 3 beams, 1.5 inches above floor, 4.5 inches apart; top row: 4 beams, 6 inches above floor, 5 inches apart). Pre-weighed water bottles were attached to the side of the cage and the tub attached to the food intake monitor was filled with sucrose pellets.
The present paradigm is similar in concept to that used by Fibiger, Phillips and colleagues to dissociate neural control of consummatory versus anticipatory feeding behaviors (Ahn & Phillips, 2002; Blackburn et al., 1987; Wilson, Nomikos, Collu, & Fibiger, 1995). The 45 min test session began with a mesh screen placed in front of the food hopper preventing rats from gaining access to the sucrose pellets, but allowing the food to be seen and smelled. After 15 min, the screen was removed and rats had ad libitum access to the sucrose pellets for 30 min. Rats were habituated to this process for 5 days or until a steady level of intake was reached. During habituation, rats were divided into two groups, trained with either sucrose or no sucrose behind the screen. After habituation days, rats were further sub-divided: saline/no sucrose (n = 8), scopolamine/no sucrose (n = 8), saline/sucrose (n=7), scopolamine/sucrose (n=7). On test day, rats received an intra-Acb infusion of saline or scopolamine (10 ug/ 0.5 ul) 3 h prior to the 45 min test session. During the initial 15 min of the test session, with the screen in front of the food source, ambulations and rears were recorded via the MedPC computer program, while an experimenter blind to treatment recorded screen approaches. Screen approaches were defined as investigatory sniffing, pulling, chewing, or examining the screen. After the initial 15 min, the screen was removed allowing rats access to the sucrose pellets for 30 min. Ambulations and rears were again recorded via MedPC. Following the 45 min session, uneaten food, food spillage, and remaining water were weighed and recorded.
Results
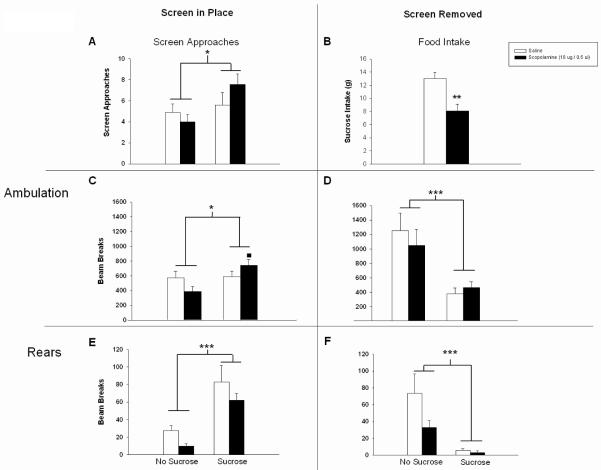
Supporting our previous work, food intake over the test session was significantly decreased in the scopolamine-treated group (t(12) = 3.545, p < 0.005; Fig 5b). Analysis of screen approaches, rearing, and ambulatory behavior during the time in which the screen prevented access to the food showed that screen approaches were not diminished in scopolamine-treated rats (F(1,26) = 0.374, n.s.; Fig 5a). As expected, there was a significant effect of training with sucrose pellets present; training in the presence of sucrose increased the number of approaches in both groups relative to rats that were never exposed to sucrose in the testing environment (F(1,26) = 5.38, p < 0.03; Fig 5a). There was no drug x group interaction for screen approaches (F(1,26) = 2.442, n.s.; Fig 5a). For ambulation behind the screen, there was no main effect of drug (F(1,26) = 0.096, n.s.; Fig 5c), but there was a significant effect of the presence of sucrose (main effect of group, F(1,26) = 5.660, p < 0.03; Fig 5c) and a drug x group interaction (F(1,26) = 4.362, p < 0.05; Fig 5c). Post-hoc comparison among means indicated a significant enhancement in ambulations in the sucrose-scopolamine group compared to the no sucrose-scopolamine group. Rearing behind the screen resulted in main effects of drug and group (F(1,26) = 5.890, p <0.03;F(1,26) = 43.874, p < 0.001; Fig 5e), but no drug x group interaction was found (F(1,26) = 0.770, n.s.; Fig 5e). This result reflects the fact that scopolamine tended to produce a mild suppression of rearing, regardless of the presence or absence of sucrose, but scopolamine-treated rats showed a proportionate increase in rearing with the sucrose present.
Figure 5.
Separation of behaviors emitted in anticipation of food, versus those emitted during access to food, with a wire screen. The left column shows behavior with the screen in place. The right column shows behaviors after the screen was removed, allowing access to the food. Training and testing in the presence of food led to a significant effect on motor behaviors 3 h following intra-Acb infusion (A-F). Scopolamine treatment significantly reduced sucrose pellet intake (B), while the number of screen approaches was unaltered when compared to saline controls (in fact, slightly but not significantly increased) (A). Ambulations behind the screen were significantly enhanced in scopolamine-treated rats trained with sucrose compared to scopolamine-treated rat, no sucrose controls (C). Rearing behavior with the screen in place, as well as with the screen removed, was somewhat suppressed by scopolamine, however no drug x group interaction was found (E & F). Error bars depict one SEM. (*p ≤ 0.05, ** p ≥ 0.01, *** p ≤ 0.001 significantly different from saline; ■ p ≤ 0.05 significantly different from no-sucrose control).
Ambulation and rearing behavior after the screen was removed did not differ between scopolamine- and saline-treated rats (ambulations: F(1,26) = 0.004, n.s.; rears: F(1,26) = 3.608, n.s.; Fig 5d & 5f respectively). As expected, there was a main effect of sucrose presence demonstrated by significant decrease in ambulation and rearing behavior due to the fact that rats were eating (ambulations: F(1,26) = 16.516, p < 0.001; rears: F(1,26) p = 29.026, p < 0.001; Fig 5d & 5f respectively). There was no drug x group interaction (ambulations: F(1,26) = 0.696, N.S.; rears: (F(1,26) = 0.568, N.S.; Fig 5d & 5f respectively). These results suggest the intra-Acb scopolamine preferentially diminishes consummatory behaviors (food intake) without altering anticipatory/preparatory motor activity and that the decrease in feeding is not secondary to non-specific motor activation.
Experiment 2: Discussion
The results of this experiment clearly show that a hypophagic dose of intra-Acb scopolamine does not alter motor behaviors antecedent to eating; specifically, there were no decreases in ambulation or screen approaches. Interestingly, scopolamine-treated rats tended to emit fewer rears in this environment. Rearing behavior, prior to screen removal, could have been influenced by the amount of time scopolamine treated rats spent in front of the screen trying to obtain food. While screen approaches (quantified as investigatory sniffing, pulling, chewing, or examining the screen) were not significantly increased by scopolamine treatment, there was a trend in this direction. These behaviors would necessarily decrease the time spent rearing, which could be the reason for the lack of enhancement of rearing behavior during the anticipatory phase by intra-Acb scopolamine. Importantly, the lack of a scopolamine-induced decrease in screen approaches would suggest that the incentive properties of the sucrose pellets were not diminished in the drug group compared to saline. Again, these results strongly oppose the hypothesis that scopolamine treatment induces a generalized negative motivational state in the rat leading to the decrease in food intake. Rather it suggests that intra-Acb scopolamine does not change (or, perhaps, slightly increases) the incentive properties of food while decreasing actual food intake.
Motor activity during the consummatory phase was unaltered by scopolamine treatment, yet food intake was still significantly decreased in the drug group compared to controls. It is not clear why the augmentation of ambulation and rearing seen in Experiment 1 was not replicated here. One likely possibility is that the ‘hyperactivity’ noted in Experiment 1 was simply the result of a shift away from long bouts of feeding. The differing arrangements of the test environments may have encouraged more ambulatory activity between feeding bouts in Experiment 1 (where the food dish was located toward the middle of the testing cage).
Finally, the present results prove that the decrease in feeding observed with scopolamine treatment was not the result of behavioral competition from hyperactivity, but an alteration in ingestive behavior once access to the food was provided.
Experiment 3: Operant responding for a food-associated Pavlovian cue
Methods
Eight commercially constructed experimental chambers (Coulbourn Instruments, Lehigh Valley, PA) enclosed in sound-attenuating cubicles, equipped with a fan to provide some masking noise, were used for the next two experiments. The chamber was made of plexiglass and aluminum, and on one of the walls were 3 stimulus lights (red, green, and yellow), a house-light, and two retractable levers. Spaced equally between the two levers was a receptacle into which 45 mg sucrose pellets could be delivered. Mounted on the receptacle was a photocell that measured nosepokes (head entries) into the food magazine. Chambers were interfaced to a personal computer via a Med-Associates (Med Associates, St Albans, VT) chassis and connector panels. Experimental events were programmed and recorded by Med PC for Windows.
Prior to the onset of training, hungry rats were habituated to the chambers for a 30 min ‘feeder/magazine’ training session. Food pellets were dispensed on a random time 30 s schedule (one pellet delivered, on average, every 30 seconds) with the house light off for the entire session. In the training phase (Pavlovian conditioning), the rats were exposed to a classical conditioning procedure involving the houselight (conditioned stimulus, CS) and sucrose pellets (unconditioned stimulus, US) with no levers present. During these sessions, the CS was presented for 10 s followed immediately by a US delivery. The session then entered an inter-trial interval (ITI), with the chamber dark, for an average of 110s (range 50 – 170 s). Daily, 30 min training sessions were conducted until an asymptotic performance on magazine entries was observed (5-8 days). Subjects were then implanted with bilateral, indwelling cannulae and following recovery (2-4) days, were retrained for several days on the previous Pavlovian conditioning procedure. Rats were habituated to the microinfusion process in the last two days of this Pavlovian retraining (see Methods).
To test the effects of scopolamine on the acquisition of conditioned reinforced (CR) responding, rats received a microinfusion of drug (scopolamine 10 μg/0.5 μl or d-amphetamine 2.5 μg/0.5 μl) or saline. Scopolamine treated rats received a 3 h pretreatment as did the saline controls. The d-amphetamine group and its controls were immediately placed in the chamber following drug infusion. The right lever was now inserted into the chamber, and upon depression of the lever, the food-associated CS (light) was presented with a probability of 1.0 for the first 5 lever-presses and then presented with a probability 0.5 for the remainder of the 30 min test session. The total number of lever presses, conditioned reinforcer presentations (light) and nosepokes were recorded automatically. To test the effects of scopolamine on the expression of already-established CR responding, the above protocol was repeated 4 days following acquisition testing (with CR testing, sans drug, on these interim days), with each rat receiving its prior saline or scopolamine treatment. A d-amphetamine probe was conducted 4 days after this test, in which rats were injected with saline or d-amphetamine and placed immediately into the operant chambers.
After completion of the CR experiment, food intake was measured following scopolamine treatment in the same set of animals. Three hours following saline or scopolamine infusion, animals were placed into the automated activity/feeding cages. Ambulations, rears, and food intake were recorded via MedPC with the experimenter measuring water intake.
To determine if scopolamine treatment could alter the d-amphetamine-induced augmentation of CR responding, a second experiment was carried out. Rats were trained as stated above, on the test day, and received an intra-Acb infusion of saline or scopolamine followed 3 h later by a saline or amphetamine infusion. Following the second drug infusion, rats were placed into the Coulbourn chambers and tested on the previously described CR schedule. Total number of lever presses, conditioned reinforcer presentations (light) and nosepokes were recorded automatically.
Results
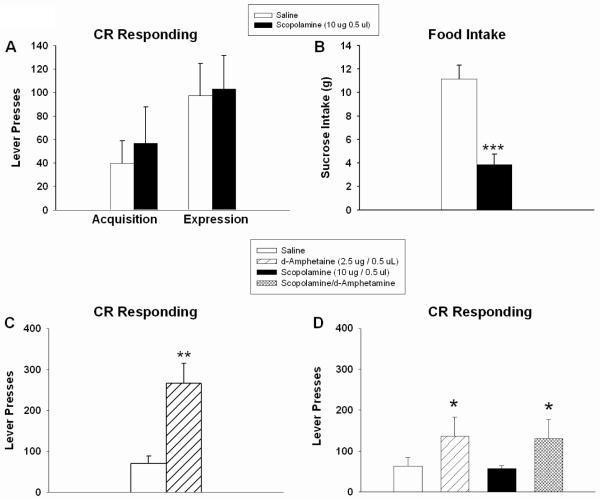
In the first experiment, looking at the effect of scopolamine-pretreatment on acquisition and expression of CR responding, no differences were found between scopolamine- and saline-treated rats in the acquisition of lever-pressing for the food-associated cue (t(14) = 0.469, n.s.; Fig 6a). In addition, 4 days after CR responding was established, there was no difference with scopolamine pretreatment on the expression of CR responding (t(13) = 0.152, n.s.; Fig 6a). To ensure the rats were not at a ceiling, intra-Acb d-amphetamine (2.5 ug / 0.5 ul) was used to potentiate CR responding. There was a main effect of d-amphetamine (t(13) = 3.595 0, p < 0.004; Fig 6c), in which number of responses increased to levels far exceeding those exhibited by scopolamine-treated rats, demonstrating that the inability to detect of enhanced CR-responding with scopolamine administration was not due to a ceiling effect. As a positive control for scopolamine action, sucrose intake was measured in these animals after a 3 h saline or scopolamine pretreatment. As shown previously, intra-Acb scopolamine significantly decreased food intake (t(14) = 4,893, p < 0.004; Fig 6b).
Figure 6.
Intra-Acb scopolamine (10 μg/ 0.5 ul) had no effect on CR acquisition or expression of responding for a food-associated cue (A). Sucrose pellet intake was significantly decreased 3 h following intra-Acb scopolamine (B). As shown in (C), CR responding was significantly increased following d-amphetamine infusion (2.5 ug / 0.5 ul) in the same set of rats used in (A) and (B). A different set of rats received a saline or scopolamine pretreatment followed by saline or d-amphetamine intra-Acb infusion. Amphetamine treatment significantly augmented CR responding, but scopolamine pretreatment had no effect on d-amphetamine potentiated responding (D). Error bars depict one SEM. (* p ≤ 0.05, ** p ≥ 0.01, *** p ≤ 0.001, significantly different from saline).
In the second experiment, to address the effect of scopolamine-pretreatment upon d-amphetamine-potentiated responding, saline or scopolamine (10 ug/ 0.5 ul) was administered 3 h prior to test session, and saline or d-amphetamine (2.5 ug/ 0.5 ul) was infused immediately prior to testing. There was no main effect of scopolamine pretreatment (F(1,13) = 0.034, n.s.; Fig 6d), but d-amphetamine significantly potentiated CR responding (main effect of d-amphetamine treatment: F(1,13) = 4,737, p < 0.05; Fig 6d). There was no pretreatment x treatment interaction (F(1,13) = 0.0008, n.s.; Fig 6d). Taken together, these results indicate that intra-Acb scopolamine treatment alters neither operant responding for a food-associated cue nor d-amphetamine-potentiation of such responding.
Experiment 3: Discussion
As mentioned previously, it has been shown that intra-Acb scopolamine produces a small, but significant decrease in lever pressing for sucrose pellets on a progressive ratio schedule (W. E. Pratt & Kelley, 2004), The progressive ratio paradigm does not distinguish between incentive properties versus reward valuation of food (‘wanting’ and ‘liking’, according to the framework elaborated by Berridge, Robinson, and colleagues (Berridge, 1996), because the pellets are eaten during the course of the test; a decrease in either property would be expected to diminish break point.
The results of the present study indicate that a hypophagic scopolamine dose does not alter responding for a food-associated cue. Saline- and scopolamine-treated rats responded almost identically to the food-associated conditioned stimulus, showing no evidence of either a performance or motivational impairment upon drug treatment. Moreover, nose pokes into the food hopper during the CR test (a measure of non-specific motor activity) were not altered by scopolamine treatment (acquisition – saline: 153.25 ± 27.880. t(14) = 1.105, n.s; scopolamine: 112.000 ± 18.612; expression – saline: 31.125 ± 7.961; scopolamine: 54.875 ± 12.335. t(14) = −1.618 n.s.. Values represent means ± SEM.). The lack of a scopolamine effect cannot be attributed to a ceiling effect on CR responding because in the same rats intra-Acb d-amphetamine infusion increased lever pressing to levels far above those seen with scopolamine. Nor can the lack of effect be due to ineffective scopolamine infusions or placements because a control experiment conducted in the same animals produced the expected decrease in food intake. In a second experiment, amphetamine treatment significantly increased lever pressing for the food-associated cue in both saline and scopolamine treated animals, however scopolamine pretreatment had no effect on responding. This is in stark contrast to the strongly suppressive effects of scopolamine on opioid-induced feeding (Perry et al., 2009; Will et al., 2006). These results show scopolamine treated rats displayed no impairment in operant responding when the reinforcer was a food-associated cue rather than food itself, and also exhibited the expected d-amphetamine-induced augmentation of CR. Taken together, these results reveal no evidence for a scopolamine-induced generalized motivational deficit. The deficit appeared only under testing conditions in which the food is actually contacted and eaten, again suggesting a specific effect on some aspect of commerce with the food (e.g. taste perception or gustatory reward).
GENERAL DISCUSSION
The results of the current study broaden our understanding of the cholinergic control of feeding behavior. To briefly summarize, the present work found in three independent experiments that intra-Acb infusion of scopolamine produced a marked and reliable decrease in food intake 3 h post infusion, but left goal-directed behaviors antecedent to ingestive behavior intact. First, analysis of feeding microstructure showed that intra-Acb scopolamine treatment greatly decreased feeding duration throughout the test session while leaving the number of bouts initiated and the latency to commence feeding unaltered, suggesting that the decrease in food consumed was not due to a decrease in the rat’s ability to identify food as a relevant incentive and to direct approach behaviors toward food. Second, an experiment designed to separately evaluate motor behavior during the anticipatory and consummatory phases of feeding demonstrated that scopolamine exclusively decreased consummatory behaviors with no alteration in anticipatory motor activity. Lastly, neither lever pressing for a food-associated conditioned stimulus, nor d-amphetamine-potentiated lever pressing for that cue, was affected by an intra-Acb scopolamine dose that markedly decreased food intake. These data suggest that muscarinic blockade initiates a cascade of events that are specific to the coding of consummatory feeding responses, but not anticipatory/preparatory behaviors or goal-seeking activities directed at a food associated cue.
The work presented here supports an emerging hypothesis regarding the neural control of feeding behavior, which proposes that the control of goal-seeking behaviors are mediated by distinct neurotransmitter systems than those governing ‘food reward’ (for discussion of this issue see (Baldo & Kelley, 2007; Barbano, Le Saux, & Cador, 2008; Berridge, 1996; Salamone, Cousins, & Bucher, 1994). The neurochemical basis for this differential control is not completely understood, and represents an active area of research. As mentioned in the Introduction, an important example is provided by the dissociable effects of dopamine and opioid manipulations in the Acb. Several studies have shown that instrumental goal-seeking behaviors are markedly influenced by DA stimulation or blockade, while measures of consummatory behavior and taste hedonics are resistant to DA manipulations but influenced by mu-opioid receptor stimulation (Bakshi VP, 1991; Bakshi & Kelley, 1993a; Berridge, Venier, & Robinson, 1989; Kelley et al., 2002; Salamone et al., 2002). Such neurochemically based dissociations have contributed to several recent theories on the neural substrates of motivation, among them the incentive-salience theory, which posits distinguishable processes underlying the ‘wanting’ (incentive value and consequent goal directed activity) and ‘liking’ (affective hedonic value) of rewards.
The current work strongly upholds this proposed dissociation and strongly suggests that cholinergic control of feeding behavior operates in the same functional realm as that of the Acb opioid system. Specifically, cholinergic antagonism does not affect responding for a food-associated cue yet decreases food intake once the rat contacts the food, just as opioid manipulations exhibit their most consistent effects in testing paradigms in which food or palatable tastants are contacted and consumed (Barbano et al., 2008; Basso & Kelley, 1999; Kelley et al., 2002). Additionally, it has been shown that scopolamine treatment blocks the feeding effect induced by the μ-agonist DAMGO, suggesting a possible cholinergic modulation of opioid-mediated feeding responses (Perry et al., 2009; Will et al., 2006). Interestingly, in a preliminary experiment, we recently found in the ‘screen test paradigm’ that systemic injection of a dopamine or opioid antagonist resulted in distinguishable profiles. The dopamine antagonist flupenthixol decreased locomotor activity with the screen in place during the anticipatory/preparatory phase, however no change in food intake was observed between drug and saline groups, while the opioid antagonist naloxone, in a similar manner to scopolamine, did not affect ambulatory activity with the screen in place, but significantly decreased the amount of food eaten during the consummatory phases (data not shown). In further support of a cholinergic-opioid interaction is the previously published study showing a reduction of preproenkephalin mRNA (the precursor to the opioid peptide enkephalin) within the striatum 24 h following intra-Acb scopolamine infusion (W. E. Pratt & Kelley, 2005). In addition to the decrease in an opioid peptide mRNA, this study additionally demonstrated a reduction in food intake, a result repeatedly shown in the present work.
At present the neural mechanisms underlying scopolamine’s central modulation of feeding behavior are poorly understood. One possibility is that the drug alters the dynamics of feeding-related acetylcholine and opioid transmission in the Acb or nearby areas of the striatum. For example, Hoebel and colleagues have shown that Acb Ach reaches its highest levels at the peak of meals (Mark, Rada, Pothos, & Hoebel, 1992) It has been pointed out that the neuronal connections and localization of muscarinic receptors on striatal cholinergic interneurons forms an interconnected network throughout the striatum, allowing for a ‘reticular’ interaction and control of vast regions of striatal tissue (Kelley, Baldo, & Pratt, 2005; Perry et al., 2009; W. E. Pratt & Kelley, 2005). In support of this idea, it has been shown that an intra-Acb scopolamine infusion decreases preproenkephalin mRNA, not only in the Acb, but throughout the striatal complex (W. E. Pratt & Kelley, 2005). This broad regulation of higher-order motor function could significantly impact the balance between anticipatory and consummatory behaviors. Hence, it is not clear that the effects of scopolamine are restricted to the Acb, but may affect transmitter dynamics throughout a broader network. It is important to note in this regard that hyperphagia induced by local mu-opioid agonist infusions is not restricted to the Acb, but is also obtained from anterior levels of dorsomedial and ventrolateral striatum. Hence, further mapping studies are needed to determine more accurately the anatomical substrates underlying cholinergic modification of feeding. Nevertheless, results of the present study strongly support the general idea that intra-Acb scopolamine alters a process, or set of processes, that are specific to the consummatory phase of feeding possibly via an interaction with the opioid system.
Finally, the information obtained in the present study could have implications for the development of appetite-control pharmacotherapeutics. A drug such as scopolamine could potentially leave the general incentive properties of food intact, but decrease the amount consumed during the meal. Hence, to exploit this therapeutically relevant mode of modulating feeding behavior, more work needs to be completed to better understand the neural mechanisms underlying scopolamine’s effects on feeding.
Acknowledgements
This research was supported by grants from National Institute on Drug Abuse (RO1 DA009311, R37DA004788, AND F31DA023775-01A1) and National Institute of Mental Health (RO1 MH074723).
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Aberman JE, Salamone JD. Nucleus accumbens dopamine depletions make rats more sensitive to high ratio requirements but do not impair primary food reinforcement. Neuroscience. 1999;92(2):545–552. doi: 10.1016/s0306-4522(99)00004-4. [DOI] [PubMed] [Google Scholar]
- Aberman JE, Ward SJ, Salamone JD. Effects of dopamine antagonists and accumbens dopamine depletions on time-constrained progressive-ratio performance. Pharmacol Biochem Behav. 1998;61(4):341–348. doi: 10.1016/s0091-3057(98)00112-9. [DOI] [PubMed] [Google Scholar]
- Ahn S, Phillips AG. Modulation by central and basolateral amygdalar nuclei of dopaminergic correlates of feeding to satiety in the rat nucleus accumbens and medial prefrontal cortex. J Neurosci. 2002;22(24):10958–10965. doi: 10.1523/JNEUROSCI.22-24-10958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi VP KA. Dopaminergic regulation of feeding behavior:II. Differential effects of amphetamine microinfusion into three striatal subregions. Psychobiology. 1991;3(19):233–242. [Google Scholar]
- Bakshi VP, Kelley AE. Feeding induced by opioid stimulation of the ventral striatum: role of opiate receptor subtypes. J Pharmacol Exp Ther. 1993a;265(3):1253–1260. [PubMed] [Google Scholar]
- Bakshi VP, Kelley AE. Striatal regulation of morphine-induced hyperphagia: an anatomical mapping study. Psychopharmacology (Berl) 1993b;111(2):207–214. doi: 10.1007/BF02245525. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Kelley AE. Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacology (Berl) 2007;191(3):439–459. doi: 10.1007/s00213-007-0741-z. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Sadeghian K, Basso AM, Kelley AE. Effects of selective dopamine D1 or D2 receptor blockade within nucleus accumbens subregions on ingestive behavior and associated motor activity. Behav Brain Res. 2002;137(1-2):165–177. doi: 10.1016/s0166-4328(02)00293-0. [DOI] [PubMed] [Google Scholar]
- Barbano MF, Le Saux M, Cador M. Involvement of dopamine and opioids in the motivation to eat: influence of palatability, homeostatic state, and behavioral paradigms. Psychopharmacology (Berl) 2008 doi: 10.1007/s00213-008-1390-6. [DOI] [PubMed] [Google Scholar]
- Basso AM, Kelley AE. Feeding induced by GABA(A) receptor stimulation within the nucleus accumbens shell: regional mapping and characterization of macronutrient and taste preference. Behav Neurosci. 1999;113(2):324–336. doi: 10.1037//0735-7044.113.2.324. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20(1):1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81(2):179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191(3):391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Venier IL, Robinson TE. Taste reactivity analysis of 6-hydroxydopamine-induced aphagia: implications for arousal and anhedonia hypotheses of dopamine function. Behav Neurosci. 1989;103(1):36–45. doi: 10.1037//0735-7044.103.1.36. [DOI] [PubMed] [Google Scholar]
- Blackburn JR, Phillips AG, Fibiger HC. Dopamine and preparatory behavior: I. Effects of pimozide. Behav Neurosci. 1987;101(3):352–360. doi: 10.1037//0735-7044.101.3.352. [DOI] [PubMed] [Google Scholar]
- Cunningham ST, Kelley AE. Opiate infusion into nucleus accumbens: contrasting effects on motor activity and responding for conditioned reward. Brain Res. 1992;588(1):104–114. doi: 10.1016/0006-8993(92)91349-j. [DOI] [PubMed] [Google Scholar]
- Hoebel BG, Avena NM, Rada P. Accumbens dopamine-acetylcholine balance in approach and avoidance. Curr Opin Pharmacol. 2007;7(6):617–627. doi: 10.1016/j.coph.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce EM, Koob GF. Amphetamine-, scopolamine- and caffeine-induced locomotor activity following 6-hydroxydopamine lesions of the mesolimbic dopamine system. Psychopharmacology (Berl) 1981;73(4):311–313. doi: 10.1007/BF00426456. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76(3):365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol. 2005;493(1):72–85. doi: 10.1002/cne.20769. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86(5):773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Bless EP, Swanson CJ. Investigation of the effects of opiate antagonists infused into the nucleus accumbens on feeding and sucrose drinking in rats. J Pharmacol Exp Ther. 1996;278(3):1499–1507. [PubMed] [Google Scholar]
- Kelley AE, Delfs JM. Dopamine and conditioned reinforcement. I. Differential effects of amphetamine microinjections into striatal subregions. Psychopharmacology (Berl) 1991;103(2):187–196. doi: 10.1007/BF02244202. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Gauthier AM, Lang CG. Amphetamine microinjections into distinct striatal subregions cause dissociable effects on motor and ingestive behavior. Behav Brain Res. 1989;35(1):27–39. doi: 10.1016/s0166-4328(89)80005-1. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Stinus L. Disappearance of hoarding behavior after 6-hydroxydopamine lesions of the mesolimbic dopamine neurons and its reinstatement with L-dopa. Behav Neurosci. 1985;99(3):531–545. doi: 10.1037//0735-7044.99.3.531. [DOI] [PubMed] [Google Scholar]
- Mark GP, Rada P, Pothos E, Hoebel BG. Effects of feeding and drinking on acetylcholine release in the nucleus accumbens, striatum, and hippocampus of freely behaving rats. J Neurochem. 1992;58(6):2269–2274. doi: 10.1111/j.1471-4159.1992.tb10973.x. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14(2-3):69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Pecina S, Berridge KC. Central enhancement of taste pleasure by intraventricular morphine. Neurobiology (Bp) 1995;3(3-4):269–280. [PubMed] [Google Scholar]
- Pecina S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic ‘liking’ for food: map based on microinjection Fos plumes. Brain Res. 2000;863(1-2):71–86. doi: 10.1016/s0006-8993(00)02102-8. [DOI] [PubMed] [Google Scholar]
- Pecina S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25(50):11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry ML, Baldo BA, Andrzejewski ME, Kelley AE. Muscarinic receptor antagonism causes a functional alteration in nucleus accumbens mu-opiate-mediated feeding behavior. Behav Brain Res. 2009;197(1):225–229. doi: 10.1016/j.bbr.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt W, Spencer R, Kelley A. Muscarinic Receptor Antagonism of the Nucleus Accumbens Core Causes Avoidance to Flavor and Spatial Cues. 2007 doi: 10.1037/0735-7044.121.6.1215. in press. [DOI] [PubMed] [Google Scholar]
- Pratt WE, Kelley AE. Nucleus accumbens acetylcholine regulates appetitive learning and motivation for food via activation of muscarinic receptors. Behav Neurosci. 2004;118(4):730–739. doi: 10.1037/0735-7044.118.4.730. [DOI] [PubMed] [Google Scholar]
- Pratt WE, Kelley AE. Striatal muscarinic receptor antagonism reduces 24-h food intake in association with decreased preproenkephalin gene expression. Eur J Neurosci. 2005;22(12):3229–3240. doi: 10.1111/j.1460-9568.2005.04489.x. [DOI] [PubMed] [Google Scholar]
- Ragnauth A, Moroz M, Bodnar RJ. Multiple opioid receptors mediate feeding elicited by mu and delta opioid receptor subtype agonists in the nucleus accumbens shell in rats. Brain Res. 2000;876(1-2):76–87. doi: 10.1016/s0006-8993(00)02631-7. [DOI] [PubMed] [Google Scholar]
- Salamone JD. The involvement of nucleus accumbens dopamine in appetitive and aversive motivation. Behav Brain Res. 1994;61(2):117–133. doi: 10.1016/0166-4328(94)90153-8. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Arizzi MN, Sandoval MD, Cervone KM, Aberman JE. Dopamine antagonists alter response allocation but do not suppress appetite for food in rats: contrast between the effects of SKF 83566, raclopride, and fenfluramine on a concurrent choice task. Psychopharmacology (Berl) 2002;160(4):371–380. doi: 10.1007/s00213-001-0994-x. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 2007;191(3):461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Bucher S. Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behav Brain Res. 1994;65(2):221–229. doi: 10.1016/0166-4328(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Mahan K, Rogers S. Ventrolateral striatal dopamine depletions impair feeding and food handling in rats. Pharmacol Biochem Behav. 1993;44(3):605–610. doi: 10.1016/0091-3057(93)90174-r. [DOI] [PubMed] [Google Scholar]
- Shannon HE, Peters SC. A comparison of the effects of cholinergic and dopaminergic agents on scopolamine-induced hyperactivity in mice. J Pharmacol Exp Ther. 1990;255(2):549–553. [PubMed] [Google Scholar]
- Will MJ, Franzblau EB, Kelley AE. Nucleus accumbens mu-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J Neurosci. 2003;23(7):2882–2888. doi: 10.1523/JNEUROSCI.23-07-02882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will MJ, Pratt WE, Kelley AE. Pharmacological characterization of high-fat feeding induced by opioid stimulation of the ventral striatum. Physiol Behav. 2006 doi: 10.1016/j.physbeh.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Wilson C, Nomikos GG, Collu M, Fibiger HC. Dopaminergic correlates of motivated behavior: importance of drive. J Neurosci. 1995;15(7 Pt 2):5169–5178. doi: 10.1523/JNEUROSCI.15-07-05169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5(6):483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Zhang M, Balmadrid C, Kelley AE. Nucleus accumbens opioid, GABaergic, and dopaminergic modulation of palatable food motivation: contrasting effects revealed by a progressive ratio study in the rat. Behav Neurosci. 2003;117(2):202–211. doi: 10.1037/0735-7044.117.2.202. [DOI] [PubMed] [Google Scholar]
- Zhang M, Gosnell BA, Kelley AE. Intake of high-fat food is selectively enhanced by mu opioid receptor stimulation within the nucleus accumbens. J Pharmacol Exp Ther. 1998;285(2):908–914. [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and fos expression. Neuroscience. 2000;99(2):267–277. doi: 10.1016/s0306-4522(00)00198-6. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Wilson CJ, Dani JA. Cholinergic interneuron characteristics and nicotinic properties in the striatum. J Neurobiol. 2002;53(4):590–605. doi: 10.1002/neu.10150. [DOI] [PubMed] [Google Scholar]