Fig. 2.
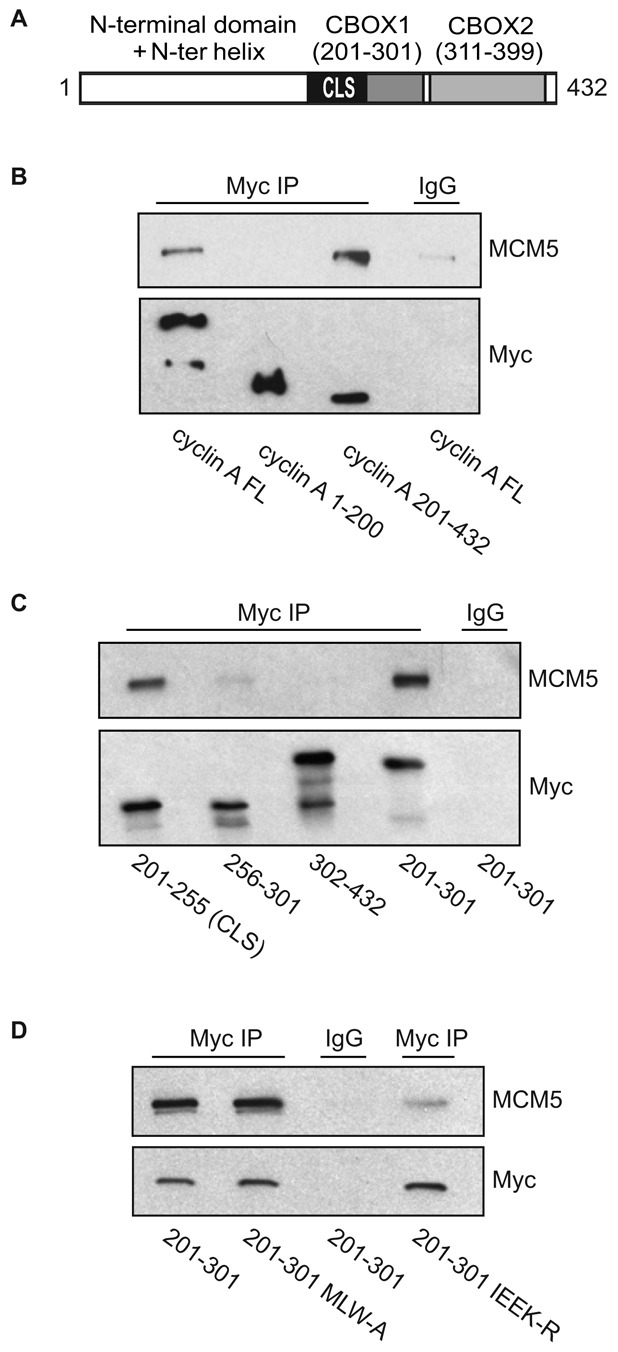
MCM5 interaction with cyclin A is CLS dependent. (A) Schematic representation of human cyclin A. The N-terminal domain (amino acids 1-200) is shown in white. The C-terminal region (amino acids 201-432) contains the cyclin domain, which can be divided into two cyclin box folds (CBOX1 and CBOX2), represented in gray. The CLS motif is highlighted in black. (B) MCM5 interacts with the C-terminus of cyclin A. CHO-K1 cells were transiently co-transfected with the indicated constructs of Myc-tagged cyclin A (FL, full length) and wild-type MCM5. Approximately 20 hours after transfection, cell lysates were prepared and subjected to immunoprecipitation (IP) with control IgG or antibody to Myc. The immunoprecipitates were separated on 10% SDS-PAGE gels and analyzed by western blotting for MCM5 and Myc-cyclin A. (C) MCM5 interacts with the CLS region of cyclin A. CHO-K1 cells were transiently co-transfected with the indicated constructs of Myc-tagged cyclin A. Analysis of MCM5 binding was carried out as in B. (D) Mutation of the cyclin A CLS disrupts MCM5 interaction. CHO-K1 cells were transiently co-transfected with wild-type MCM5 and Myc-tagged cyclin A amino acids 201-301 containing a wild-type CLS, the hydrophobic patch mutant CLS (MLW-A) or mutant CLS (IEEK-R), as indicated. Analysis of MCM5 co-immunoprecipitation was carried out as in B.
