Abstract
Background
In order to provide reliable tissue material for malignant mesothelioma (MM) studies, we re-evaluated biopsies and autopsy material from 61 patients with a diagnosis of MM from the period of 1980-2002.
Methods
Basic positive (Calretinin, EMA, Podoplanin, Mesothelin) and negative (CEA, Ber-Ep4) immunohistochemical (IHC) marker reactions were determined. If needed, more markers were used. Histological diagnoses were made by three pathologists. Survival data were calculated.
Results
49 cases (80%) were considered being MM by a high degree of likelihood, five more cases possible MM. Of the remaining seven cases, three were diagnosed as adenocarcinoma, three as pleomorphic lung carcinoma, in one peritoneal case a clear entity diagnosis could not be given. One of the possible MM cases and two of the lung carcinoma cases had this already as primary diagnoses, but were registered as MM.
With a sensitivity of 100%, Calretinin and CEA were the most reliable single markers. The amount of MM cells with positive immunoreactivity (IR) for Podoplanin and Mesothelin showed most reliable inverse relation to the degree of atypia.
In the confirmed MM cases, there had been applied either no IHC or between one and 18 markers.
The cases not confirmed by us had either lacked IHC (n = 1), non-specific markers were used (n = 4), IR was different (n = 1), or specific markers had not shown positive IR in the right part of the tumour cells (n = 3).
46 of the 49 confirmed and three of the not confirmed cases had been diagnosed by us as most likely MM before IHC was carried out.
Conclusions
In order to use archival tissue material with an earlier MM diagnosis for studies, histopathological re-evaluation is important. In possible sarcomatous MM cases without any positive IR for positive MM markers, radiology and clinical picture are essential parts of diagnostics. IHC based on a panel of two positive and two negative MM markers has to be adapted to the differential diagnostic needs in each single case. New diagnostic tools and techniques are desirable for cases where IHC and other established methods cannot provide a clear entity diagnosis, and in order to improve MM treatment.
Background
Modern molecular techniques applied on tissue specimens have increased the interest in using archival material for research purposes. The risk of wrong diagnosis by doing so might be high due to the lack of immunohistochemical (IHC) evaluation at the time of diagnosis.
Malignant mesothelioma (MM) is a tumour that traditionally has been considered being difficult to diagnose histologically in a substantial subset of cases. To our knowledge, the extent of misdiagnosis of MM in archival material has not been studied systematically earlier, with the exception of one recent study [1].
MM is a highly aggressive tumour defined as derived from mesothelial cells [2,3]. It is considered an almost incurable disease. Its molecular profile indicates that most of the known genes for radio- and chemoresistance are overexpressed [4,5].
MM has traditionally been divided into epithelial, sarcomatous and mixed types, where the less common mixed and sarcomatous variants show a poorer survival. Sometimes a desmoplastic type is added, which may be diagnosed as either epithelial, mixed or sarcomatous type [6,7]. For the mixed type MM, International Mesothelioma Panel (IMP)/World Health Organization (WHO) require the presence of arbitrarily at least 10% of either an epithelial or a sarcomatous component [3]. According to recent studies, median survival after diagnosis is 4.5 to 17 months, depending on histological type, tumour stage, performance status and treatment, and other factors as gender and age [8-11].
In Norway, registered new MM cases have continuously increased from five cases in the period of 1958-62 to 382 cases in the period of 2003-07. In the last period, 88% of registered cases were histologically verified. By adding the cytologically diagnosed cases the rate of morphologically diagnosed cases reached nearly 100% (97.5% of 360 cases in the period of 2001-05) [12-14]. However, in its recent guidelines the European Respiratory Society (ERS)/European Society of Thoracic Surgeons (ESTS) Task Force does not recommend making a MM diagnosis based on cytology alone because of the high risk of diagnostic error [15].
Before IHC became a part of routine pathology in the end of the 1980s/beginning of the 1990s, and especially before more MM-specific positive IHC markers were introduced a few years later, it could be difficult or even impossible to differentiate MM from other malignant tumours, such as primary or metastatic adenocarcinomas (AC) in a part of the cases. Around 1995, antibodies against Wilms tumour protein (WT) 1, around 1998, against Calretinin and around 2005, against Podoplanin/D2-40 were introduced into routine diagnostics as new positive MM markers [16-22].
The introduction of such more specific positive markers, in combination with distinctive negative markers has significantly improved the possible accuracy of MM diagnosis. However, there is no reliable single MM IHC marker. The IMP/WHO and the International Mesothelioma Interest Group (IMIG) recommendations on MM IHC diagnostics comprise a panel of at least two positive and two negative antibodies [3,23].
This study aimed primarily at establishing histopathological diagnoses on archival tissue material from patients diagnosed as MM, in order to get a reliable basis for further studies using recent histological and IHC criteria. Furthermore, we wished to find out how the availability and choice of IHC markers may have influenced MM diagnosis.
Methods
The archival material in this study has been used in two previous studies where verification of the histological MM diagnoses was mandatory [11,24]. Use of the material was approved by the Regional Ethical Committee.
Paraffin-embedded tissue material from 73 patients taken between 1980 and 2002 was received from 19 Norwegian departments of pathology. Primary MM diagnoses had been given mostly based on tumour biopsies, and/or in a few cases on autopsy material. Tissue specimen volumes varied from a few mm3 in needle biopsies to some cm3 in autopsy material. Clinical and radiological data were fragmentary, and limited to those received in written form by the primarily diagnosing pathologists in the histology requisitions. After having received additional clinical information, tumour localisation was adjusted to both pleura and peritoneum in three cases, and to scrotum in one case, compared with earlier data [11,24]. From two of the four patients with MM both in pleura and peritoneum, autopsy material from both localisations was disposable, from the others the biopsies were only from one site.
From the paraffin blocks, Hematoxylin-Eosin-Saffron (HES) stained slides were made. For 61 patients (84%), the samples showed a sufficient amount of remaining tumour tissue suitable for diagnosis by the planned IHC procedure. Preliminary, exclusively HES-based tentative diagnoses were established. Basic histological parameters were determined on HES-stained slides. Mitoses were counted per so-called high-power fields (HPF), constituted by 40 times of objective and 10 times of ocular magnification.
After having determined optimal antibody dilution individually for each marker, by applying established IHC protocols from routine pathology and in the remaining cases by testing optimal dilutions, IHC was performed by the standardised Dako Cytomation protocol DAKO REAL™ EnVision™ with peroxidase/diaminobencidine and chromogen, rabbit/mouse [25]. The minimum procedure included four positive (Calretinin, Epithelial Membrane Antigen (EMA) membranous, Mesothelin, Podoplanin) and two negative (carcino-embryonic antigen (CEA), Ber-Ep4) markers, and in a subset of cases, if considered necessary, additional IHC markers (each case including, as a minimum, Hector Battifora Mesothelial Cells (HBME) 1 and Thrombomodulin as positive and CD15 and Sialosyl-TN as negative markers, before applying Mesothelin and Podoplanin) and Alcian Blue staining. In order to reach a reliable MM diagnosis, in five cases of epithelial type MM (No. 30, 39, 62, 74, 75) (12%) and in two cases of mixed MM (nr. 11 and 25) (25%), this second set of four basic IHC markers was applied. Reasons for this were too weak and/or not typical immunoreactivity (IR).
Slides were evaluated in multiple steps defined by the used basic and additional antibodies, by two pathologists (E. Larsson, H. Sandeck) independently, finally by a third pathologist (H. Willén), before establishing a diagnosis without access to the previous histological diagnoses. After each step, the diagnosis or eventual markers to add were determined by consensus. Our MM selection criteria included the histological MM variants and subtypes according to the current WHO MM classification and clearly positive IHC staining in at least a part of the tumour tissue, or absence of staining by the mentioned positive and negative markers, respectively [3]. For the diagnosis of a sarcomatous type or component, we regarded a predominant spindle cell pattern as indispensable [26]. A definitive entity diagnosis was only given when there was a high degree of likelihood for the entity based on morphology and IHC, with positive IR for at least one positive MM marker (Table 1).
Table 1.
Immunohistochemical markers used for malignant mesothelioma diagnosis in this study
| IHC marker | Antigen type | Clone | Producer | Code No. | Dilution |
|---|---|---|---|---|---|
| Positive markers | |||||
| Calretinin | Calcium-binding protein | polyclonal | Zymed | 18-0211 | 1: 1,500 |
| EMA | HMFG protein | E-29 | Dako | M0613 | 1: 750 |
| Thrombomodulin | endothelial cell transmembrane glycoprotein | 1009 | Dako | M0617 | 1: 50 |
| HBME-1 | mesothelial cell membrane protein | HBME-1 | Dako | M3505 | 1: 50 |
| Mesothelin | cell surface glycoprotein | 5B2 | Novocastra | NCL-L-MESO | 1: 10 |
| Podoplanin | transmembrane mucoprotein | n.s., monoclonal | Angiobio | 11-003 | 1: 50 |
| CK5/6 | IMF | D5/16 B4 | Dako | M7237 | 1: 80 |
| CK7 | IMF | OV-TL12/30 | Dako | M7018 | 1: 1,000 |
| CK AE1/AE3 | IMF (pan-CK cocktail, subfamilies A and B) | AE1 and AE3 | Dako | N1590 | 1: 75 |
| CK KL1 | IMF (pan-CK cocktail) | KL1 | Serotec | MCA144H | 1: 50 |
| Negative markers | |||||
| monoclonal CEA | Gold 1 epitope | II-7 | Dako | M7072 | 1: 200 |
| polyclonal CEA | CEA and CEA-like proteins | polyclonal | Dako | A0115 | 1: 13,000 |
| Ber-Ep4 | transmembrane glycoprotein | Ber-Ep4 | Dako | M 0804 | 1: 200 |
| CD15 | Lewis X carbohydrate antigen | C3D-1 | Dako | M0733 | 1: 25 |
| Sialyl-TN | Sialosyl-Tn1 glycoprotein | HB-STn1 | Dako | M0899 | 1: 100 |
| TTF-1 | nuclear transcription factor | 8G7G3/1 | Dako | M3575 | 1: 100 |
| Others | |||||
| CK20 | IMF | Ks 20.8 | Dako | M7019 | 1: 200 |
| CD10 | neutral endopeptidase 24.11 | SS2/36 | Dako | M0727 | 1: 50 |
| CD34 | single-chain transmembrane protein | QBEnd 10 | Dako | M7165 | 1: 100 |
| CD68 | glycosylated lysosomal membrane protein | KP1 | Dako | M814 | 1: 3,000 |
| CD99 | MIC2 gene products | 12E7 | Dako | M3601 | 1: 100 |
| Bcl-2 | apoptosis inhibitor | 124 | Dako | M0887 | 1: 200 |
| SMA | microfilaments | 1A4 | Dako | M0851 | 1: 300 |
| Desmin | IMF | D33 | Dako | M0760 | 1: 100 |
| S-100 | Calcium-binding proteins | polyclonal | Dako | Z0311 | 1: 3,000 |
| HMB-45 | part of neuraminidase-sensitive glycoconjugate in melanosomes | HMB45 | Dako | M0634 | 1: 100 |
| HHF-35 | muscle actin | HHF 35 | Dako | M0635 | 1: 200 |
| MIB1 | nuclear protein, proliferation marker | Ki-67/MIB-1 | Dako | M7240 | 1: 200 |
Abbreviations:
IMF - intermediate filament; EMA - Epithelial Membrane Antigen; HMFG - Human Milk Fat Globule; HBME - Hector Battifora Mesothelial (Human Mesothelial Cell Antigen); CK - Cytokeratin; AE - Anti-Epithelial; KL1 - Keratin L1; CD - Cluster of Differentiation; TTF - Thyroid Transcription Factor; Bcl - B-cell lymphoma; SMA - Smooth Muscle Actin; HMB - Human Melanoma Black; HHF - Human Heart Fibroblasts/myofibroblasts; MIB - Molecular Immunology Borstel; Ki - Kiel
Cases with negative IR were coded 0 and those with positive IR were classified into quartiles (H. Sandeck). Sensitivity (number of MM cases with positive IR divided by total number of MM cases that underwent IHC for the marker in question) and specificity (number of carcinoma reference cases with negative IR divided by total number of carcinoma reference cases for the marker in question) were calculated for positive and negative MM IHC markers.
Survival data were obtained from the Cancer Registry of Norway. These and the microscopic data were processed in the statistical software SPSS, v. 16.0 (Producer: SPSS Inc., IBM Company, Chicago, USA), with survival being a dependent variable. Survival dependent on each single histological and IHC parameter and on a few basic clinical data was determined, and visualised by Kaplan-Meier plots. Parametric linear (Pearson) and non-parametric (Kendall's tau-b, Spearman's rho) bivariate correlations to survival were analysed with regard to significance. A multivariate linear model was calculated.
Results
Re-classification
Of the 61 evaluable cases, 49 (80%; 40 men, 9 women) were diagnosed as MM, 40 (82%) as epithelial and 8 (16%) as mixed type. Of the mixed type cases, seven were localised in pleura, two of them were autopsy cases that showed secondary spreading via diaphragm into peritoneum. The remaining case was located in scrotum. All of the mixed MM types occurred in men. In one pleural case (No. 7) (2%), typing was undetermined between epithelial and mixed because of ca. 10% of sarcomatous component. All four cases of MM affection in both pleura and peritoneum were observed in men. A summary of our results is shown in Table 2 [see also Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6].
Table 2.
Classification of cases
| Female | Male | Total | |
|---|---|---|---|
| Total number of evaluable cases | 11 | 50 | 61 |
| Validated MM | 9 | 40 | 49 (80%) |
| Epithelial/mixed/between epithelial and mixed type | 9/0/0 | 31/8/1 | 40/8/1 |
| Pleura/mediastinum/pericardium | |||
| - Epithelial type | 6/1/0 | 24/0/1 | 30/1/1 |
| - Mixed type | 0/0/0 | 5/0/0 | 5/0/0 |
| - Between epithelial and mixed type | 0/0/0 | 1/0/0 | 1/0/0 |
| Peritoneum/pleura and peritoneum/scrotum | |||
| - Epithelial type | 2/0/- | 3/2/1 | 5/2/1 |
| - Mixed type | 0/0/- | 0/2/1 | 0/2/1 |
| Others | 2 | 10 | 12 (20%) |
| Adenocarcinoma, lung | 0 | 2 | 2 |
| Adenocarcinoma, peritoneum | 1 | 0 | 1 |
| Pleomorphic carcinoma | 0 | 3 | 3 |
| No definitive entity diagnosis, amongst them MM as one differential diagnosis |
1 0 |
5 5 |
6 5 |
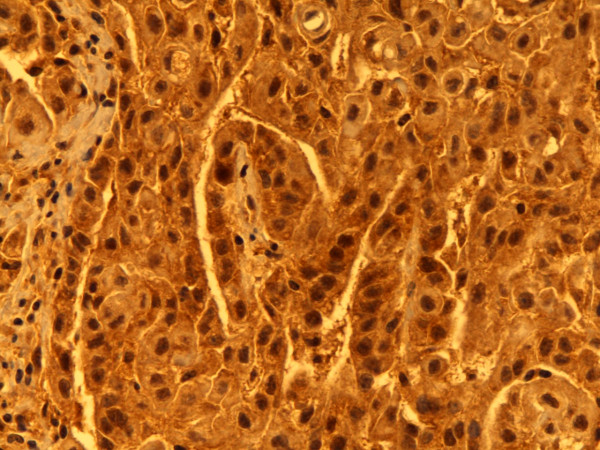
Figure 1.
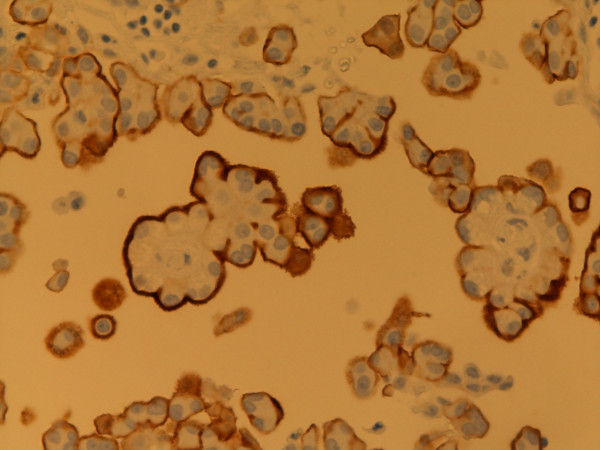
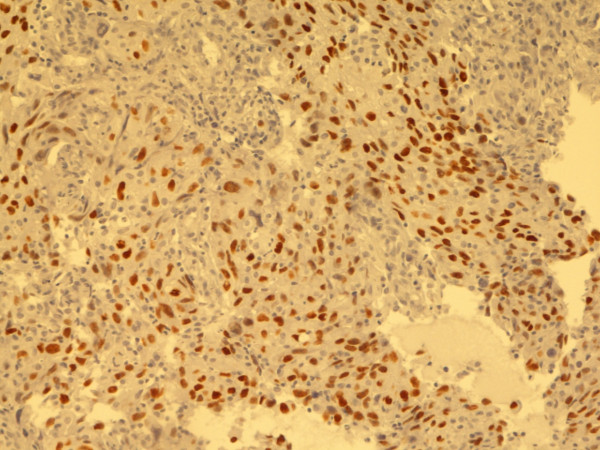
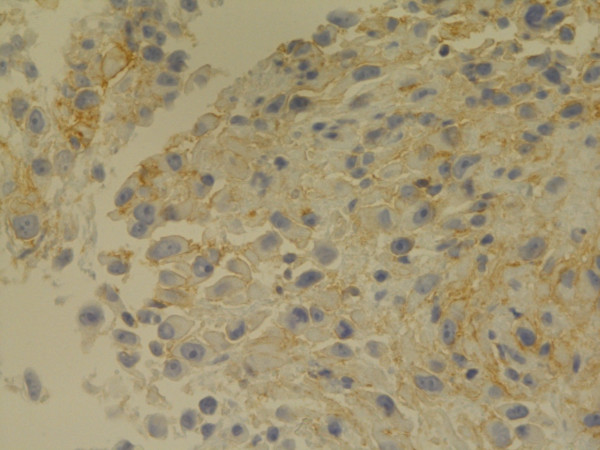
Calretinin. Predominantly nuclear, less cytoplasmatic staining. Epithelial type MM, pleura. Biopsy, 400×. Pat. no. 1.
Figure 2.
Calretinin. Predominantly nuclear staining. Mixed type MM, pleura. Autopsy material, 400×. Pat. no. 69.
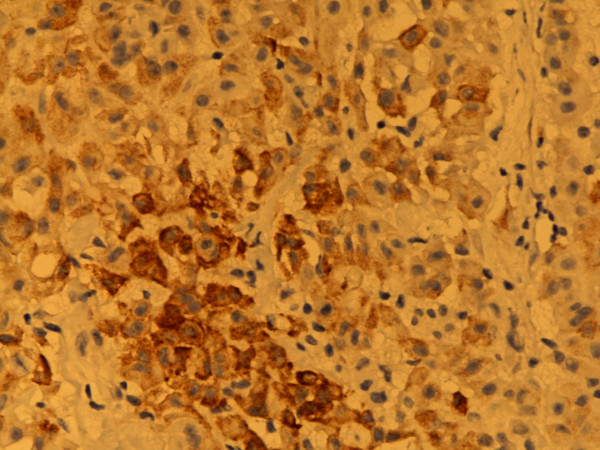
Figure 3.
Epithelial membrane antigen. Various intensities of staining from negative to strongly positive, predominantly membranous. Epithelial type MM, pleura. Biopsy. 400×. Pat. no. 1.
Figure 4.
Epithelial membrane antigen. Partially cytoplasmatic, predominantly membranous staining. Mixed type MM, epithelial component, pleura. Biopsy, 400×. Pat. no. 33.
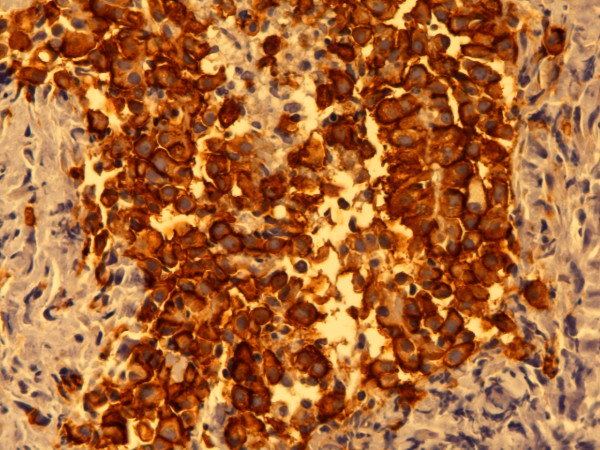
Figure 5.
Mesothelin. Membranous staining. Epithelial type MM, pleura. Biopsy, 400×. Pat. no. 1.
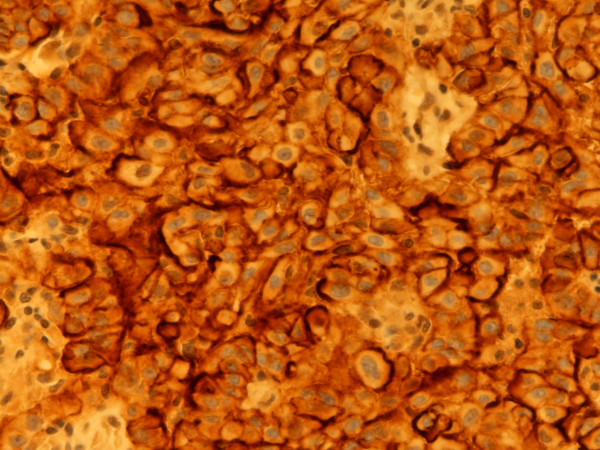
Figure 6.
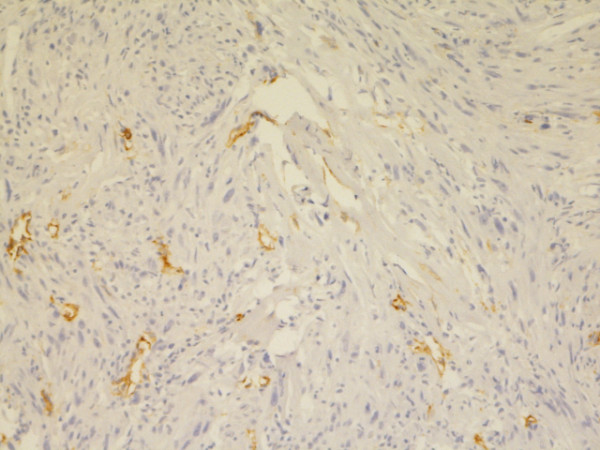
Podoplanin. Microvilli staining. Epithelial type MM, peritoneum. Biopsy, 400×. Pat. no. 52.
One earlier diagnosis of epithelial type had to be changed to mixed type when autopsy material was included (No. 11) (related to [11,24]). The remaining 12 cases (20%; 10 men, 2 women), represented various malignancies, where the primary MM diagnosis was either abolished (n = 7) or set more in doubt (n = 5). Of the five cases in which MM yet was considered possible, one was without IHC in primary diagnosis (No. 5); in the other four cases, the IHC profile was not considered being sufficient for a MM diagnosis (No. 57, 32, 63, 12). In four of these cases, sarcomatous MM and not furthermore specified sarcoma were differential diagnoses, while there were no sufficient indications for sarcomatous carcinoma (No. 5, 57, 32, 63) (Tables 3, 4) [see Figure 7, Figure 8, Figure 9, Figure 10, Figure 11 and Figure 12].
Table 3.
Diagnostic certainty dependent on localisation of primary tumour
| Localisation | Confirmed MM | Possible MM | No MM | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fem. | Male | Total | Fem. | Male | Total | Fem. | Male | Total | |
| Lung/pleura | 6 | 30 | 36 | 0 | 5 | 5 | 0 | 5 | 5 |
| Mediastinum (n.s.) | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pericardium | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pleura and peritoneum | 0 | 4 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Peritoneum | 2 | 3 | 5 | 0 | 0 | 0 | 2 | 0 | 2 |
| Scrotum | - | 2 | 2 | - | 0 | 0 | - | 0 | 0 |
| Sum | 9 | 40 | 49 | 0 | 5 | 5 | 2 | 5 | 7 |
n.s.: localisation not furthermore specified
Data are based on primarily registered MM.
Table 4.
Possible causes of diagnostic discordance related to time of diagnosis
| Possible causes | Pat. ID | Sex | Year | Our tentative HES diagnosis | Primarily registered MM diagnoses changed to: |
|---|---|---|---|---|---|
| Right histological diagnosis (without use of IHC) not accepted by clinicians, registered as MM in Cancer Registry |
5 3 6 |
M M M |
1986 1988 1988 |
MM/LMS MM MM/C |
undetermined: sarcoma, lung or pleura/sarcomatous MM, pleura AC, lung pleomorphic carcinoma, lung |
| No IHC | 9 | M | 1991 | MM/C | pleomorphic carcinoma, lung |
| Use of non-specific markers | 57 72 31 49 |
M M M F |
1989 1996 1998 1998 |
MM MM/C Mal. tu. S/LMS? |
undetermined: sarcoma, lung or pleura/sarcomatous MM, pleura AC, lung pleomorphic carcinoma, lung undetermined: sarcoma/sarcomatoid carcinoma, peritoneum |
| Differences in IR1 | 58 | F | 1999 | MM/C | AC, peritoneum |
| Not appropriate conclusions2 | 32 63 12 |
M M M |
1999 2001 2002 |
MM Ben./MM? Mal. Mel. |
undetermined: sarcoma, lung or pleura/sarcomatous MM, pleura undetermined: sarcoma, lung or pleura/sarcomatous MM, pleura undetermined: AC, lung > MM, pleura |
1 between the primary laboratory and our laboratory, in addition misinterpretation of IR of another marker (one case: 58; Ber-Ep4 from negative IR in primary diagnosis to positive IR in our diagnosis, EMA positive IR only in cytoplasm)
2 based on a few relevant MM markers that showed no typical MM pattern
AC - adenocarcinoma; C - carcinoma; S - sarcoma; LMS - leiomyosarcoma; Mal. - malignant; Tu. - tumor; Ben. - benign; Mel. - melanoma
None of these cases had been referred to a second opinion.
All cases were registered as MM, partially despite of histological diagnoses.
Primary histological diagnoses were:
Pat. 5: Malignant mesenchymal tumour, MM possible, no epithelial component seen
Pat. 3: Poorly differentiated carcinoma most likely, MM less likely
Pat. 6: Carcinoma most likely
Pat. 9: MM
Pat. 57: MM likely
Pat. 72: MM likely
Pat. 31: MM possible
Pat. 49: MM
Pat. 58: MM
Pat. 32: MM most likely
Pat. 63: MM, sarcomatous type
Pat. 12: MM
Figure 7.
Adenocarcinoma, lung. TTF-1. Biopsy, 200×. Pat. no. 3.
Figure 8.
Pleomorphic giant cell carcinoma, lung. TTF-1. Biopsy, 200×. Pat. no. 9.
Figure 9.
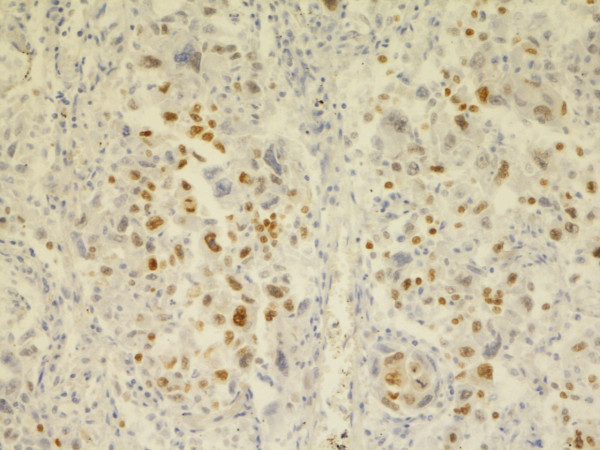
Possible sarcomatous MM, chest wall. Positive IR for Calretinin in some spindle cells. Biopsy, 200×. Pat. no. 57.
Figure 10.
Possible epithelial MM, lung/pleura. Membranous positive IR for Podoplanin in some cells. Biopsy, 400×. Pat. no. 12.
Figure 11.
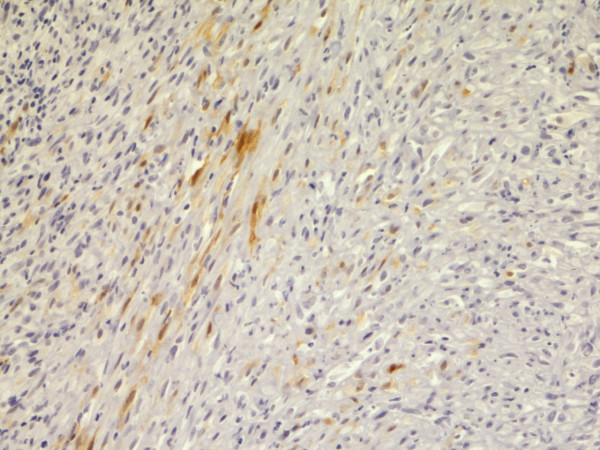
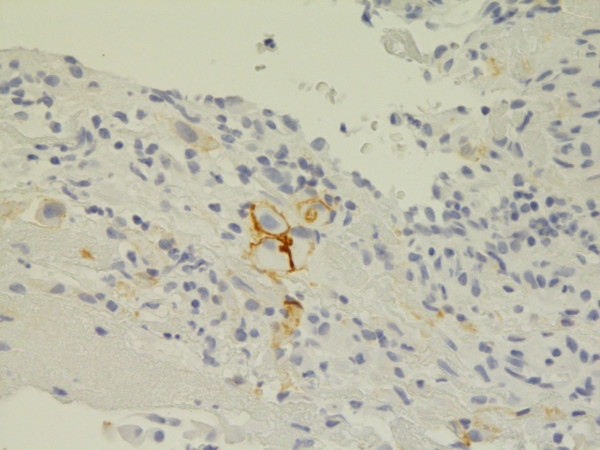
Possible sarcoma, pleura/lung. Positive IR for Podoplanin nearly only in lymphatic endothelium. Biopsy, 200×. Pat. no. 5.
Figure 12.
Possible sarcoma, pleura/lung. CD99. Biopsy, 400×. Pat. no. 32.
In one of the possible MM cases (No. 5) and two of the lung carcinoma cases (No. 3, 6) primary histological diagnoses were in accordance to our diagnoses, but had been registered as MM in the Cancer Registry. This was done apparently by clinicians who did not accept the histological diagnoses (No. 3, 6), or the likelihood consideration expressed in one of them (No. 5). Thus, in a stronger sense, only nine previous MM diagnoses (9/61, 15%), namely histological ones, were not confirmed by us. Four of them (7%) were classified as carcinomas, five (8%) as undetermined with possible MM in four of them (Table 4).
11 of the 12 mentioned, with respect to MM not confirmed cases had been diagnosed primarily in university departments of pathology. In eight of the 49 confirmed cases and in one of the not confirmed cases no university department was ever involved in primary diagnosis. Institutional affiliation of primary diagnosis is shown in Table 5.
Table 5.
Institutions in which primary histopathological diagnostics were performed
| Institution where primary diagnosis was determined (n = 61) | ||
|---|---|---|
| Non-university dept. | University dept. | |
| Original diagnostic unit | 19 | 42 |
| Consultation with another, university department | 8 | 5 |
| Re-evaluation in another, university department |
2 | 1 |
| Primary histological diagnosis confirmed | 18 (95%) | 34 (81%) |
| Primary histological diagnosis not confirmed | 1 (5%) | 8 (19%) |
In all but one (No. 9, pleomorphic carcinoma of the lung) of the eight cases with diagnoses based on autopsy material, primary MM diagnoses were confirmed. In six cases the basis marker set of Calretinin, EMA, CEA and Ber-Ep4 was sufficient for diagnosis, there were five epithelial MM and one mixed MM. In one case, more IHC markers were considered being necessary, here our diagnosis was mixed type MM.
Sensitivity and specificity of IHC markers
Based on the five pulmonary carcinoma cases, specificity was lower than sensitivity for Podoplanin, EMA and monoclonal CEA, but 100% for Calretinin and Mesothelin. Concerning sensitivity, the most reliable single markers amongst the six markers were Calretinin and CEA. However, in two of 49 MM cases Calretinin showed positive IR only in a few tumour cells (Table 6). No difference in MM IR for monoclonal and polyclonal CEA was detected, although inflammatory cells gave a positive IR for polyclonal, and not for monoclonal CEA.
Table 6.
Sensitivity and specificity, confirmed MM cases
| Marker | Sensitivity, MM | Specificity, against carcinoma | ||||
|---|---|---|---|---|---|---|
| Positive MM markers | positive IR, number | positive IR, % | literature data, % | negative IR, number % | negative IR, % | literature data, % |
| Calretinin, nucleus | 49/49 | 100 | 821 96.42 |
5/5 | 100 | 851;2 |
| EMA, cell membrane | 43/49 | 87.8 | 843 | 3/5 | 60 | 563 |
| Podoplanin, cell membrane | 44/46 | 95.6 | 864 | 2/3 | 66.7 | 1004 |
| Mesothelin, cell membrane | 44/48 | 91.7 | 1005 | 4/4 | 100 | 615 |
| Thrombomodulin, cell membrane | 7/7 | 100 | 611 | 2/5 | 40 | 801 |
| HBME-1, cell membrane or cytoplasm | (4+2)/8 | 75 | 851 | (2-1)/5 | 20 | 431 |
| CK5/6 | 3/3 | 100 | 831 | 3/5 | 60 | 851 |
| Negative MM markers | negative IR, number | negative IR, % | positive IR, number | positive IR, % | ||
| CEA, monoclonal | 49/49 | 100 | 811 | 1/5 | 20 | 971 |
| Ber-Ep4 | 40/49 | 81.6 | 801 | 3/5 | 60 | 901 |
| CD15 | 7/7 | 100 | 84.96 | 1/5 | 20 | 816 |
| Sialosyl-TN | 6/7 | 85.7 | 76.56 | 1/5 | 20 | 816 |
| TTF-1 | 3/3 | 100 | 721 | 5/5 | 100 | 1001 |
Specificity, own results: against AC and pulmonary pleomorphic carcinoma.
Comparison to some selected data from literature.
1 King et al. 2006, meta-analysis of literature data, MM with epithelioid areas against pulmonary AC [27].
2 Takeshima et al. 2009: sensitivity epithelial/mixed MM 96.4%/90% [1]. Yaziji et al. 2006: sensitivity 95%, specificity against AC 87%, both with 10% positive cutoff [28].
1, 2 Calretinin IR not specified.
3 Wick et al. 1990, related to epithelial MM and pulmonary AC [29].
4 Ordóñez 2005, related to epithelial MM and AC [21].
5 Ordóñez 2003a, related to epithelial MM and pulmonary AC [30].
6 Brockstedt et al. 2000, related to epithelial/mixed MM and AC [31].
Marker expression in relation to grade of atypia
Two of 49 MM cases (4%) showed the lowest degree of cytological atypia in the material, grade 1 and 1.5, and three of them (6%) high atypia, grade 3. The expression of Podoplanin and Mesothelin in the grade 3 cases was in all cases low compared to the grade 1 till 2 cases.
In five cases there were a few MM cells, near 0%, with positive IR for Ber-Ep4, in four cases some more, but less than 25% of MM cells. IR for CEA was negative in all cases (not shown). Of the four positive markers, Calretinin and EMA were most expressed even in poorly differentiated MM (Table 7).
Table 7.
Histological grading of atypia and immune reaction for main MM markers
| Grade of atypia (n = 49) |
Cases (n) |
Percent (%) |
Calretinin, nucleus (n = 49) |
Epithelial membrane antigen, cell membrane (n = 49) |
Podoplanin (n = 47) |
Mesothelin (n = 48) |
Ber-Ep4 (n = 49) |
|---|---|---|---|---|---|---|---|
| 1 | 0 | 0 | - | - | - | - | - |
| 1.5 | 2 | 4 | 4 4 |
1 3 |
4 3 |
4 4 |
0 0 |
| 2 | 25 | 51 | 2.84 (1...4) |
2.00 (0...4) |
2.65 (1...4) |
3.46 (0...4) |
.08 (0...1) |
| 2.5 | 19 | 39 | 2.26 (near 0...4) |
2.16 (near 0...4) |
1.74 (0...4) |
3.11 (0...4) |
.11 (0...1) |
| 3 | 3 | 6 | 2 3 1; 1 |
2 2 2; 1 |
1 1 1 |
2 1 1 |
0 0 0 |
Number of MM cells with positive IR, semiquantitatively on a 0...4 scale (steps of 25%). For grades 2 and 2.5, average values and overall minimum-maximum ranges are indicated. IR for CEA was negative in all of the cases.
No obvious differences in IHC staining intensity and distribution were seen between biopsy and autopsy material, even if there were prominent signs of autolysis in the latter one, or related to the age of the tissue material (not shown). However, there were only four cases with both biopsy and autopsy material, with partially insufficient amount of the former one. A systematic comparison of marker expression in these cases as in [32] was not performed.
Use of IHC in primary diagnosis in the confirmed MM cases
In at least 26 cases (53%) between one and 18 IHC markers had been applied (Table 8). In all but one of the confirmed cases, the positive marker combination Calretinin and EMA could have been replaced by the combination Podoplanin and Mesothelin, leading to the same diagnosis. In one case (autopsy, No. 70) which showed only a few nuclei with positive IR for Calretinin and positive membranous IR for EMA in less than 25% of cells, the combination Podoplanin and Mesothelin, with IR in two and three quartiles of tumour cells, respectively, would have been a better option. However, in a case of MM of tunica vaginalis testis (biopsy, No. 25) with only a few cells showing the same kind of IR for Calretinin and EMA, IR for Podoplanin and Mesothelin were completely negative.
Table 8.
Application of IHC in primary diagnosis in confirmed cases (some patterns, partially overlapping cases)
| Year | Confirmed cases | IHC/markers in primary diagnostics |
|---|---|---|
| 1980-2002 | 49 (100%) | |
| 19 (39%) | No IHC performed | |
| 4 (8%) | No information available about eventual IHC use | |
| 26 (53%) | IHC, 1 till 18 markers | |
| No specific positive MM markers | ||
| 1988-1999 | 7 (14%) | CEA, Vimentin and Pan-Cytokeratin |
| Broad spectrum of non-specific markers | ||
| 1996, 1997 | 2 (4%) | 15/18 markers, 1st one incl. positive marker HBME-1, otherwise no specific positive MM markers |
| Positive MM markers | ||
| 1998-2002 | 8 (16%) | Calretinin, in all but one (1998) of cases combined with other markers, two cases with another positive marker - Thrombomodulin and HBME-1, respectively (n = 1-7) |
| Negative MM markers | ||
| 1988-2002 | 17 (35%) | CEA, in all but one (1990) of cases combined with other markers (n = 1-18) |
| 1996, 2002 | 2 (4%) | CD15, combined with, amongst other markers, CEA and Ber-Ep4 (n = 15, 7) |
| 1996-2002 | 10 (20%) | Ber-Ep4, combined with other markers (n = 4-15) |
Use of IHC in primary diagnosis in the not confirmed MM cases
Four of the 12 not confirmed cases from between 1986 and 1991 lacked IHC. In four cases from between 1989 and 1998, non-specific IHC markers were applied. One case from 1999 (No. 58) showed different IR for one crucial negative MM marker, Ber-Ep4, which was described as negative in primary diagnosis, but which showed positive IR in our IHC staining. The positive marker EMA showed positive IR, but only in cytoplasm. In the remaining three cases from between 1999 and 2002 which all are of undetermined entity, combinations of relevant IHC markers including Calretinin, EMA and Ber-Ep4 were used, but Calretinin showed negative IR and EMA positive IR in cytoplasm. In five of the six undetermined cases, MM is still one of the differential diagnoses (Tables 2, 4) [see Additional file 1].
Tentative diagnosis without IHC
In 46 of the 49 cases confirmed by us (94%) we previously also had diagnosed MM as most likely based on HES slides only. In the remaining three cases, MM was a differential diagnosis together with carcinoma. In eight of the 12 not confirmed cases (67%), MM was one of our tentative differential diagnoses. In three of these cases (25%), MM was our only tentative diagnosis. Two of the latter cases remained undetermined, one case showed most likely pulmonary AC after IHC (Table 4).
Survival
Overall median survival was 12.5 months, with almost no difference between men and women (12.2 and 12.5 months, respectively). Pleural MM survival ranged from 0 to 44 months, with a median of 12.5 months. Mediastinal, pericardial and scrotal MM (1/1/2 cases) survival was 2.5, 4, 6 and 10 months, respectively. The longest median survival, 51.8 months, was observed in peritoneal MM, with a minimum of 5.5 months in men and 31.5 months in women.
Survival was in the statistic median and mean poorer when IHC Mesothelin expression was reduced, this was also true for Calretinin in nuclei and even more for Podoplanin, which was the only IHC marker showing significant bivariate correlation [11]. Mixed type, presence of spindle cells, high cellular atypia, more than one mitosis per 10 HPF, presence of atypical mitoses, necrosis, presence of regional lymph node and/or distant metastases and elevated age at diagnosis were correlated with poorer survival. Peritoneal MM was correlated with longer survival than MM in all other localisations. All of these parameters, except the number of atypical mitoses, showed significant bivariate correlation with survival, too (Table 9).
Table 9.
Survival-related single factors, tendencies of correlation
| Factor | Relation to survival, clear tendency | Significant (only) in bivariate correlation with survival (two-tailed), level |
|---|---|---|
| IHC markers, semiquantitatively, when increasing number of cells with positive IR: | ||
| Calretinin, nucleus | ↑ | - |
| Calretinin, cytoplasm | ↔ | - |
| EMA, cell membrane | ↔ | - |
| EMA, cytoplasm | ↔ | - |
| Podoplanin, cell membrane | ↑ | 0.001 |
| Mesothelin, cell membrane | if 0-50%: ↓ 51-100%: ↑ |
- |
| Ber-Ep4, general | if 1-25%: (↓) | - |
| Histological type | ||
| Epithelial | ↑ | 0.05 (non-parametric) |
| Mixed | ↓ | 0.01 (non-parametric) |
| Histological markers | ||
| a) when increasing: | ||
| Degree of cellular atypia | ↓ | 0.001 |
| Spindle cells, semiquantitatively | if 0: ↑; otherwise: ↓ | 0.001 (non-parametric) |
| Number of mitoses (10 HPF) | if 0 or 1: ↑; otherwise:↓ | 0.05 (Spearman's rho) |
| Number of atypical mitoses (10 HPF) | if 0: ↑; otherwise: ↓ | - |
| Necrosis | ↓ | 0.01 |
| Mucus, cytoplasm, number of cells | if high: (↓) | - |
| Mucus, extracellular, number of cells | ↔ | - |
| Accompanying inflammation, intensity | ↔ | - |
| Accompanying inflammation, focality | ↔ | - |
| b) if present: | ||
| MM spreading, perineural | (↔) | - |
| MM spreading, intralymphangic | (↔) | - |
| MM spreading, intralymphangic and venoles | (↓) | - |
| No/no evident MM spreading | ↑ | - |
| Accompanying inflammation, active | (↔) | - |
| Accompanying inflammation, non-active | ↔ | - |
| Localisation of primary tumour | ||
| Pleura | ↓ | - |
| Peritoneum | ↑ | 0.01 (parametric) 0.05 (non-parametric) |
| Pleura and peritoneum | (↓) | - |
| Mediastinum, not specified | (↓) | - |
| Pericardium | (↓) | - |
| Scrotum | (↓) | - |
| Metastasis | 0.05 (non-parametric) | |
| yes | ↓ | |
| no/not known | ↑ | |
| Sex | - | |
| Female | ↔ | - |
| Male | ↔ | - |
| Age at time of diagnosis, when increasing: | ↓ | 0.01 |
In parentheses: very few cases
Survival is given per April 21, 2008.
Multivariate linear analysis revealed a model to which only localisation in peritoneum, cellular atypia and necrosis contributed significantly, namely at <0.05 levels (at 0.001 level, 0.031 and 0.032, respectively), not the other parameters that had shown significant bivariate correlations with survival (0.2 till 0.9 levels in multivariate analysis).
Women were only represented in the primary diagnosis age groups of 45 till 59 years, men in all age groups from 45 till 79 years. There was a tendency of longer survival in men then in women in the age groups of 45-54 years. No relevant differences in survival could be observed between men and women, when results were stratified according to localisation in pleura or peritoneum. All four cases of MM affection in both pleura and peritoneum were observed in men. Concerning epithelial type MM, no survival difference was seen between men and women, mixed type MM was only observed in men.
Survival in lung AC was 1.5 and 7.5 months, in pleomorphic lung carcinoma 1; 3.5 and 13 months, in possible peritoneal sarcoma/sarcomatoid carcinoma 2 months, in possible pulmonary/pleural sarcoma/sarcomatous MM (No. 5, 57, 32) 3, 1 and 1 months, in possible pulmonary AC/pleural MM 2 months and in peritoneal AC at least 100 months (related to Table 4).
Discussion
In this study, we re-evaluated a Norwegian tissue material with an earlier diagnosis of MM which was regarded as potentially suitable to further molecular studies aiming at a deeper understanding of MM biology [4,11,24].
All patients were donors to the Norwegian Janus serum bank, as the only applied selection criterion of the MM material [33].
Our material is not representative concerning MM type distribution, as there have been diagnosed more mixed and sarcomatous types in larger newer studies (Roberts et al. 2001: 73% epithelial/11% mixed/10% sarcomatous/6% desmoplastic; Borasio et al. 2008: 67% epithelial/23% mixed/10% sarcomatous), so far as one supposes that similar diagnostic criteria were applied, which is not necessarily the case [32,9]. However, when including the four cases of possible sarcomatous type in our study, proportions would be more similar (n = 40/8/4; 77%/15%/8%). MM organ distribution seems to reflect the overall incidence [12].
Due to scarce material, some relevant antibodies as WT-1, h-Caldesmon, and others, as well as specific histochemical reactions on glycoproteins and hyaluronic acid could not be applied, despite of their diagnostic value [34-36]. More extensive diagnostics including genetic testing, fluorescence in situ hybridisation (FISH) and electron microscopy (EM) in order to possibly reach a final entity diagnosis in the unclear cases, were not carried out, since this was not a concern in this study where the main point was to exclude all insecure and non-MM cases by a high degree of likelihood [37,23,39].
Our choice of Calretinin, EMA, CEA and Ber-Ep4 was determined by their diagnostic value, as recently established by Brockstedt et al. who singled out CEA and Calretinin as the most informative markers in differential diagnosis between MM and AC [31]. Their next most valuable markers were CD15 and EMA, while Ber-Ep4 was fifth. The reason to choose Ber-Ep4 instead of CD15 as one basic negative marker was to test whether Ber-Ep4 really could replace CD15 in this constellation, as it according to our knowledge apparently has been used much more. However, direct comparison was only possible in seven cases where also IHC on markers no. 5-8 (HBME-1, Thrombomodulin, CD15 and Sialosyl-TN) was performed. As there was partial positive IR for Ber-Ep4 in two verified MM cases, but negative IR for CD15 in all of the seven verified cases, we too experienced that CD15 was a more reliable marker than Ber-Ep4. In four of the seven cases (no. 11, 25, 39, 62), CK5/6 and TTF-1 were considered necessary for confirming the diagnosis. Mesothelin was chosen in order to compare IR on MM tissue with serum levels and to evaluate it as an IHC marker, Podoplanin for the latter reason [4,11,30,40,20,21].
WHO/IMP demand a minimum of 10% sarcomatous cells to define a mixed MM. In order to reduce MM typing problems, it has been proposed that at least 30-40% of a sarcomatous component should be present to call it a mixed type [41]. This would also be appropriate in order to avoid taking sarcoma-like stromal reactions as true sarcomatous MM components. However, in small biopsies, even such a component ratio may happen to be not representative at all. Which cytological and histological patterns are considered to represent a sarcomatous MM component or type is also varying [26,42]. Such variations in opinions, approaches and definitions may cause significant differences in the MM type frequencies making it difficult or impossible to compare different studies.
We detected a 15% discrepancy of former histological MM diagnosis. This could be explained by either 1) lack of IHC (n = 1), 2) use of non-specific IHC markers (n = 4), 3) differences in IR (n = 1) or 4) not appropriate conclusions based on appropriate markers (n = 3) influencing the primary diagnosis.
The case of the first group was from the period before 1998 when no specific IHC markers were available for actual differential diagnostics in routine pathology. Today it would be quite uncommon not to use IHC at all in MM diagnostics, at least in industrialized Western countries. At the same time, this illustrates the problems that may occur in countries with a less developed health care system where IHC might not be affordable.
As to the non-specific markers used in the second group that covers a period until 1998, this was due to the lack of more specific markers.
Differences in IR as in the third group and not appropriate conclusions as in the fourth group may occur also today. The former may be reduced by the use of standardized equipment and workflow in IHC laboratories, e.g., by IHC staining machines.
In two cases where there had been used non-specific markers in primary IHC (No. 31, 72), the later (1999) introduced marker TTF-1 was crucial to establish the pulmonary carcinoma diagnosis. This also happened in three of the cases that had lacked IHC (No. 3, 6, 9) (table 4) [43].
Sarcoma, not further specified, was a differential diagnosis in five of the six cases with undetermined re-evaluation diagnosis, together with sarcomatous MM of pleura/lung in four of them. This reflects the potential diagnostic difficulty in a part of the sarcomatous lesions of pleura and lung even when IHC is available. In the one CK-negative (no. 32) and in the four CK-positive (no. 5, 49, 57, 63) spindle cell tumour cases, Comparative Genomic Hybridisation (CGH) might have allowed distinguishing between sarcomatous MM and other entities [37].
The high percentage of cases in which university departments were involved amongst the cases not confirmed by us (8/9, 89%, with respect to histology) may be partially due to the generally high rate of MM diagnoses in which a university department was involved (52/61, 85%). Amongst these cases, there were no consultation cases sent from non-university departments. Apparently, difficult cases were sent from non-university departments to university departments for a second opinion, or primary diagnosis. However, in none of the not confirmed cases, a second opinion was asked for.
Whereas there does not seem to exist poorly differentiated epithelial type MM without any expression of one or more of such positive markers, as, e.g., Calretinin, WT-1, Mesothelin or Podoplanin, the complete absence of IR for such markers is allowed for the sarcomatous type by the above mentioned definition, as far as there is a positive IR for broad-spectrum cytokeratins. This would indeed mean that, with increasing dedifferentiation, specific mesothelial markers disappear, while cytokeratins are preserved even in poorly differentiated sarcomatous MM. However, in cases with completely negative IR for mesothelial markers, there would not be any sufficient histological evidence for the presence of MM. The clinical and radiological picture would then play a major role in tentative diagnosis.
In a study by Blobel et al., all forms of MM expressed CK 8 and 18, and most of them CK 7 and 19, the same CK profile as in AC [44]. In addition, CK 5 was expressed in all epithelial and most of the mixed types. CK 4, 6, 14 and 17 were also present in variable amounts (see also [45]). The authors concluded that MM has to be defined as a variant of carcinoma. Histologically, this is indeed a reasonable conclusion even from today's point of view [46]. Sarcomatous MM could then be regarded as mesothelial sarcomatoid carcinoma. On the other hand, several sarcomas also express cytokeratins.
In our material, 19 MM diagnoses (39%) from the whole period (1980-2002) which were established without any use of IHC could be confirmed by us. All of them were also tentatively diagnosed by us as most likely MM in HES only slides. Apparently, the microscopic pictures in these cases were sufficient in order to recognize epithelial or mixed type MM. Related to our final diagnoses, in about 94% of the cases of epithelial MM, our preliminary, tentative HES-based entity diagnoses were correct. There would remain about 6% of diagnostic failure regarding the epithelial subtype alone. Most often this would concern the differential diagnosis between MM and AC, which may have therapeutic implications. In the differential diagnosis of sarcomatoid lesions, without IHC, there would be a much higher error rate when the pathological diagnosis is based only on HE or HES stained slides. This underlines the value of IHC in MM diagnosis, especially if there is any doubt concerning the HES picture.
Before more specific MM IHC markers were introduced, in the confirmed MM cases, marker combinations consisting of CEA and Pan-cytokeratin or Vimentin were applied. Here, negative IR on CEA and the morphological picture seem to have been crucial for the MM diagnosis, since there may be positive IR of both Pan-cytokeratin and Vimentin in carcinomas and sarcomas. Specific positive MM markers were not available until approximately 1998 when Calretinin was introduced in Norway.
All of our confirmed cases showed at least focally positive IR for Calretinin. However, there were no cases amongst the not confirmed cases in which missing Calretinin IR would have been the only exclusion criterion [see Additional file 1].
A basic IHC marker combination of two positive (e.g., Calretinin, EMA membranous) and two negative (Ber-Ep4, CEA) markers was well applicable to distinguish most of epithelial MM from other differential diagnostic possibilities. When it is necessary to distinguish MM from pulmonary AC, and when a synovial sarcoma can be ruled out, positive IR for TTF-1 would be useful for a pulmonary carcinoma diagnosis. With the exception of EMA, all of the mentioned markers are among the mesothelial and epithelial markers focused on by the WHO panel [3]. Meanwhile, positive membranous IR for EMA scored high as a positive MM, or mesothelial marker in the study of Brockstedt et al. that is based on a large MM material, and it is also mentioned as usually negative in benign mesothelial proliferations in the recent IMIG recommendations [31,23]. EMA is also a recommended marker in the recent ERS/ESTS guidelines [15].
Based on our results, only Calretinin and CEA showed 100% sensitivity (Table 6). In two cases, only a few MM cells showed positive nuclear IR for Calretinin. One of them (no. 25) was a mixed MM of tunica vaginalis testis. In a study of Winstanley et al. on 20 tunica vaginalis testis cases, all of them showed negative IR for CEA and positive IR for Calretinin, but only 16 (80%) nuclear IR for Calretinin [47]. Our second case (no. 75) was an epithelial MM of pleura that also showed only a few cells with positive membranous IR for EMA.
Because of the predominating purpose of re-evaluation, the used tissue material was not set up against a material of non-MM tumours relevant in differential diagnosis. Thus, our results on specificity of MM IHC markers cannot be generalized, although the results do reflect the historical development of MM IHC diagnostics.
For epithelial MM, IMP and IMIG recommend a combination of at least two positive and two negative markers, and a broad spectrum CK mix. They point out that the preferred markers depend on the experience of the laboratory, and that more mesothelial, epithelial, vascular, and malignant melanoma markers can become necessary if the results are not conclusive [6,23]. In our study, in the first IHC step, we followed these recommendations, except for the broad spectrum CK antibodies. However, we used three broad spectrum CK antibodies (AE1, AE3, KL1) together with other markers in some cases of possible sarcomatous MM where negative IR on these antibodies would indicate that the tumour was not a MM. For a diagnosis of epithelial and mixed MM, more than two positive and negative markers were considered being necessary in 12% and 25%, respectively.
Concerning the choice of IHC markers, Marchevsky and Wick point out the substantial value that odds rate calculations, systematic reviews and meta-analysis may have for the development of evidence-based guidelines for MM diagnosis [48]. This is even true if there are several possible applicable "optimal" marker combinations, dependant on the experience of the laboratory and the specific case.
A recent study on accuracy of pathological MM diagnosis based on a more than 5-fold larger Japanese material (382/73) set up as interdisciplinary re-evaluation shows with 15.8% (47/298) of all cases diagnosed by IHC a similar scale of diagnoses classified as "definitively not/unlikely" MM, compared with 11.5% (7/61) in our study [1]. The authors defined 80.5% (240/298) of the IHC cases as "probable/definite" MM, while the corresponding amount in our study was virtually the same, 80.3% (49/61). However, in the Japanese study, even cytological material and not furthermore processed histological slides were included; IHC was only performed in 80.6% (308/382) of the cases. As in our study, diagnostic accuracy was lower in sarcomatoid lesions. While there was no agreement in diagnoses in as much as 22.4% of all female pleural and 72.2% of all female peritoneal cases, the respective numbers in our study are 0% (0/6) and 50% (2/4).
Detailed data on exposition to asbestos or other possible MM risk factors were not available to us. By using census data from 1970 where occupations had been classified into having high, moderate or little/no asbestos exposure and expanding them to the 1960 and 1980 censuses, we have earlier made estimations on the possible asbestos exposure of nearly the same patient group (n = 47, two patients less than in the current study). Occupational asbestos exposure was likely in 12 patients, all men. However, these data could not be validated [11].
Survival analysis revealed no surprising data in respect of recent oncological knowledge on MM or malignant tumours in general. The presence of atypical mitoses alone, irrespective of the number of mitoses, could possibly be an independent negative prognostic factor, but this has to be verified or falsified on a larger material. Any sex preponderance in MM survival could not be seen in our material. The results of multivariate linear analysis may not be representative because of the too low number of cases for this purpose.
Conclusions
Our results underline the need of histopathological re-evaluation of tissue biopsies with an earlier diagnosis of MM, especially those given before the introduction of at least one of the more specific positive MM markers that showed a good diagnostic reliability in our cases. In our opinion, a reliable IHC-based definition of the sarcomatous MM type has been absent for cases with negative IR for mesothelium-specific markers. We have considered such cases not being sufficient for a definitive pathological MM diagnosis, but if clinical and radiological findings support a MM diagnosis in such a setting, nevertheless, a MM diagnosis may be established. Differential diagnoses in our material were adenocarcinoma, pleomorphic and sarcomatoid carcinoma and sarcoma. The WHO/IMP and IMIG recommendations of at least two positive and two negative IHC markers can be affirmed by our study, as far as sensitivity is concerned. The marker choice has to be adapted to the specific differential diagnostic needs in each single case. In difficult cases, it may be useful to refer to a second opinion.
In six cases, despite of relatively extensive IHC application, we were not able to give a clear-cut entity diagnosis, which may highlight the need of novel diagnostic tools as biomarkers and other molecular analyses, as well as new criteria. Such new tools will also be needed for the more personalized approaches for cancer treatment in the future.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
HS participated in the design of the study, established histological diagnoses, processed the data and drafted the manuscript.
ODR participated in the design of the study and reviewed the manuscript.
KK provided data from the Cancer Registry of Norway and reviewed the manuscript.
HW established histological diagnoses and reviewed the manuscript.
EL participated in the design of the study, established histological diagnoses and reviewed the manuscript.
All authors read and approved the final manuscript.
Supplementary Material
Comparison of primary and re-evaluation IHC/HC in the 12 cases where MM diagnosis was not confirmed, chronologically.
Contributor Information
Helmut P Sandeck, Email: helmut.sandeck@stolav.no.
Oluf D Røe, Email: oluf.roe@stolav.no.
Kristina Kjærheim, Email: kristina.kjaerheim@kreftregisteret.no.
Helena Willén, Email: helena.willen@akademiska.se.
Erik Larsson, Email: erik.larsson@genpat.uu.se.
Acknowledgements
We very much thank all the departments of pathology in Norway that allowed us to use their archival material for our study.
Thanks to Prof. Dr. Dr. K. Kayser, Heidelberg, Prof. Dr. R. Moll, Marburg, and Prof. Dr. H. Popper, Graz, for useful information and discussion.
For the laboratory work, we very much thank U. Granli and B. Ytterhus, Institute of Laboratory Medicine, Women's and Children's Diseases, Norwegian University of Science and Technology, Trondheim, Norway.
References
- Takeshima Y, Inai K, Amatya VJ, Gemba K, Aoe K, Fujimoto N, Kato K, Kishimoto T. Accuracy of pathological diagnosis of mesothelioma cases in Japan: clinicopathological analysis of 382 cases. Lung Cancer. 2009;66:191–197. doi: 10.1016/j.lungcan.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Kayser K. Analytical Lung Pathology. Berlin etc., Springer-Verlag; 1992. [Google Scholar]
- Churg A, Roggli V, Galateau-Sallé F, Cagle PhT, Gibbs AR, Hasleton PhS, Henderson DW, Vignaud JM, Inai K, Praet M, Ordóñez NG, Hammar SP, Testa JR, Gazdar AF, Saracci R, Pugatch R, Samet JM, Weill H, Rusch V, Colby TV, Vogt P, Brambilla E, Travis WD. In: Pathology and genetics of tumours of the lung, pleura, thymus and heart. Travis WD, Brambilla E, Müller-Hermelink HK, Curtis CH, editor. WHO/IARC. Lyon, IARC Press; 2004. Mesothelioma; pp. 128–136. [Google Scholar]
- Røe OD. Malignant mesothelioma: virus, biomarkers and genes: a translational approach. PhD Thesis. Norwegian University of Science and Technology, Faculty of Medicine, Institute of Cancer Research and Molecular Medicine & Institute of Laboratory Medicine, Children's and Women's Health, Trondheim. 2008.
- Røe OD, Anderssen E, Helge E, Pettersen CH, Olsen KS, Sandeck H, Haaverstad R, Lundgren S, Larsson E. Genome-wide profile of pleural mesothelioma versus parietal and visceral pleura: the emerging gene portrait of the mesothelioma phenotype. PLoS ONE. 2009;4:e6554. doi: 10.1371/journal.pone.0006554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (ed) Pathology of malignant mesothelioma. Springer, London; 2006. [Google Scholar]
- Butnor KJ, Sporn TA, Ordóñez NG. Association of Directors of Anatomic and Surgical Pathology (ADASP) Recommendations for the reporting of pleural mesothelioma. Virchows Arch. 2007;450:15–23. doi: 10.1007/s00428-006-0301-7. [DOI] [PubMed] [Google Scholar]
- Okada M, Mimura T, Ohbayashi C, Sakuma T, Soejima T, Tsubota N. Radical surgery for malignant pleural mesothelioma: results and prognosis. Interact Cardiovasc Thorac Surg. 2008;7:102–106. doi: 10.1510/icvts.2007.166322. [DOI] [PubMed] [Google Scholar]
- Borasio P, Berruti A, Bille A, Lausi P, Levra MG, Giardino R, Ardissone F. Malignant pleural mesothelioma: clinicopathologic and survival characteristics in a consecutive series of 394 patients. Eur J Cardiothorac Surg. 2008;33:307–313. doi: 10.1016/j.ejcts.2007.09.044. [DOI] [PubMed] [Google Scholar]
- Ceresoli GL, Locati LD, Ferreri AJM, Cozzarini C, Passoni P, Melloni G, Zannini P, Bolognesi A, Villa E. Therapeutic outcome according to histologic subtype in 121 patients with malignant pleural mesothelioma. Lung Cancer. 2001;34:279–287. doi: 10.1016/S0169-5002(01)00257-4. [DOI] [PubMed] [Google Scholar]
- Røe OD, Creaney J, Lundgren S, Larsson E, Sandeck H, Boffetta P, Nilsen TI, Robinson B, Kjrheim K. Mesothelin-related predictive and prognostic factors in malignant mesothelioma: a nested case-control study. Lung Cancer. 2008;61:235–243. doi: 10.1016/j.lungcan.2007.12.025. [DOI] [PubMed] [Google Scholar]
- Cancer Registry of Norway. Cancer in Norway 2007 - Cancer incidence, mortality, survival and prevalence in Norway. Oslo. 2008. http://www.kreftregisteret.no/no/Registrene/Kreftstatistikk
- Ulvestad B, Kjaerheim K, Møller B, Andersen A. Incidence trends of mesothelioma in Norway, 1965-1999. International Journal of Cancer. 2003;107:94–98. doi: 10.1002/ijc.11357. [DOI] [PubMed] [Google Scholar]
- Larsen IK, Småstuen M, Johannesen TB, Langmark F, Parkin DM, Bray F, Møller B. Data quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer. 2009;45:1218–1231. doi: 10.1016/j.ejca.2008.10.037. [DOI] [PubMed] [Google Scholar]
- Scherpereel A, Astoul P, Baas P, Berghmans T, Clayson H, de Vuyst P, Dienemann H, Galateau-Salle F, Hennequin C, Hillerdal G, Le Péchoux C, Mutti L, Pairon JC, Stahel R, van Houtte P, van Meerbeeck J, Waller D, Weder W. ERS/ESTS Task Force Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J. 2010;35:479–495. doi: 10.1183/09031936.00063109. [DOI] [PubMed] [Google Scholar]
- Walker C, Rutten F, Yuan X, Pass H, Mew DM, Everitt J. Wilms' tumor suppressor gene expression in rat and human mesothelioma. Cancer Res. 1994;54:3101–3106. [PubMed] [Google Scholar]
- Amin KM, Litzky LA, Smythe WR, Mooney AM, Morris JM, Mews DJY, Pass HI, Csaba K, Rodeck U, Rauscher FJ III, Kaiser LR, Albeida SM. Wilms' tumor 1 susceptibility (WT1) gene products are selectively expressed in malignant mesothelioma. Am J Pathol. 1995;146:344–356. [PMC free article] [PubMed] [Google Scholar]
- Gotzos V, Vogt P, Celio MR. The calcium binding protein calretinin is a selective marker for malignant pleural mesotheliomas of the epithelial type. Pathol Res Pract. 1996;192:137–147. doi: 10.1016/S0344-0338(96)80208-1. [DOI] [PubMed] [Google Scholar]
- Doglioni C, Dei AP, Laurino L, Iuzzolino P, Chiarelli C, Celio MR, Viale G. Calretinin: a novel immunocytochemical marker for mesothelioma. Am J Surg Pathol. 1996;20:1037–1046. doi: 10.1097/00000478-199609000-00001. [DOI] [PubMed] [Google Scholar]
- Kimura N, Kimura I. Podoplanin as a marker for mesothelioma. Pathol Int. 2005;55:83–86. doi: 10.1111/j.1440-1827.2005.01791.x. [DOI] [PubMed] [Google Scholar]
- Ordóñez NG. D2-40 and podoplanin are highly specific and sensitive immunohistochemical markers of epithelioid malignant mesothelioma. Hum Pathol. 2005;36:372–380. doi: 10.1016/j.humpath.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Sonne SB, Herlihy AS, Hoei-Hansen CE, Nielsen JE, Almstrup K, Skakkebæk NE, Marks A, Leffers H, Rajpert-De Meyts E. Identity of M2A (D2-40) antigen and gp36 (Aggrus, T1A-2, podoplanin) in human developing testis, testicular carcinoma in situ and germ-cell tumours. Virchows Arch. 2006;449:200–206. doi: 10.1007/s00428-006-0223-4. [DOI] [PubMed] [Google Scholar]
- Husain AN, Colby TV, Ordóñez NG, Krausz T, Borczuk A, Cagle PT, Chirieac LR, Churg A, Galateau-Salle F, Gibbs AR, Gown AM, Hammar SP, Litzky LA, Roggli VL, Travis WD, Wick MR. Guidelines for pathologic diagnosis of malignant mesothelioma: a consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2009;133:1317–1331. doi: 10.5858/133.8.1317. [DOI] [PubMed] [Google Scholar]
- Kjærheim K, Røe OD, Waterboer T, Sehr P, Rizk R, Dai HY, Sandeck H, Larsson E, Andersen A, Boffetta P, Pawlita M. Absence of SV40 antibodies or DNA fragments in prediagnostic mesothelioma serum samples. International Journal of Cancer. 2007;120:2459–2465. doi: 10.1002/ijc.22592. [DOI] [PubMed] [Google Scholar]
- Dako Corporation. REAL™ EnVision™ Detection System, Peroxidase/DAB+, Rabbit/Mouse. Kode K5007. 3. 2008. http://www.dako.dk/prod_downloadpackageinsert.pdf?objectid=105995004 objectid= 1059 95004 Glostrup. [Google Scholar]
- Laga AC, Allen T, Bedrossian C, Murer B, Cagle PhT. In: Color atlas and text of pulmonary pathology. Cagle PT, editor. Philadelphia, Lippincott Williams & Wilkins; 2005. Diffuse malignant mesothelioma. [Google Scholar]
- King JE, Thatcher N, Pickering CA, Hasleton PS. Sensitivity and specificity of immunohistochemical markers used in the diagnosis of epithelioid mesothelioma: a detailed systematic analysis using published data. Histopathology. 2006;48:223–32. doi: 10.1111/j.1365-2559.2005.02331.x. [DOI] [PubMed] [Google Scholar]
- Yaziji H, Battifora H, Barry TS, Hwang HC, Bacchi CE, McIntosh MW, Kussick SJ, Gown AM. Evaluation of 12 antibodies for distinguishing epithelioid mesothelioma from adenocarcinoma: identification of a three-antibody immunohistochemical panel with maximal sensitivity and specificity. Mod Pathol. 2006;19:514–23. doi: 10.1038/modpathol.3800534. [DOI] [PubMed] [Google Scholar]
- Wick MR, Loy T, Mills SE, Legier JF, Manivel JC. Malignant epithelioid pleural mesothelioma versus peripheral pulmonary adenocarcinoma: a histochemical, ultrastructural, and immunohistologic study of 103 cases. Hum Pathol. 1990;21:759–66. doi: 10.1016/0046-8177(90)90036-5. [DOI] [PubMed] [Google Scholar]
- Ordóñez NG. Value of mesothelin immunostaining in the diagnosis of mesothelioma. Modern Pathology. 2003;16:192–197. doi: 10.1097/01.MP.0000056981.16578.C3. (2003a) [DOI] [PubMed] [Google Scholar]
- Brockstedt U, Gulyás M, Dobra K, Dejmek A, Hjerpe A. An optimized battery of eight antibodies that can distinguish most cases of epithelial mesothelioma from adenocarcinoma. Am J Clin Pathol. 2000;114:203–209. doi: 10.1309/QHCA-8594-TA7P-0DVQ. [DOI] [PubMed] [Google Scholar]
- Roberts F, McCall AE, Burnett RA. Malignant mesothelioma: a comparison of biopsy and postmortem material by light microscopy and immunohistochemistry. J Clin Pathol. 2001;54:766–770. doi: 10.1136/jcp.54.10.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langseth H, Gislefoss RE, Martinsen JI, Stornes A, Lauritzen M, Andersen Aa, Jellum E, Dillner J. The Janus Serum Bank. From sample collection to cancer research. Oslo, Cancer Registry of Norway. 2009. http://www.kreftregisteret.no/Global/29-01-2010%20CIN_2008_Special_Issue_Janus_Web.pdf
- Comin CE, Dini S, Novelli L, Santi R, Asirelli G, Messerini L. h-Caldesmon, a useful positive marker in the diagnosis of pleural malignant mesothelioma, epithelioid type. Am J Surg Pathol. 2006;30:463–469. doi: 10.1097/00000478-200604000-00006. [DOI] [PubMed] [Google Scholar]
- Comin CE, Saieva C, Messerini L. h-caldesmon, calretinin, estrogen receptor, and Ber-EP4: a useful combination of immunohistochemical markers for differentiating epithelioid peritoneal mesothelioma from serous papillary carcinoma of the ovary. Am J Surg Pathol. 2007;31:1139–1148. doi: 10.1097/PAS.0b013e318033e7a8. [DOI] [PubMed] [Google Scholar]
- Kayser K, Böhm G, Blum S, Beyer M, Zink S, André S, Gabius HJ. Glyco-and immunohistochemical refinement of the differential diagnosis between mesothelioma and metastatic carcinoma and survival analysis of patients. J Pathol. 2001;193(2):175–80. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH772>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Knuuttila A, Jee KJ, Taskinen E, Wolff H, Knuutila S, Anttila S. Spindle cell tumours of the pleura: a clinical, histological and comparative genomic hybridization analysis of 14 cases. Virchows Arch. 2006;448:135–141. doi: 10.1007/s00428-005-0059-3. [DOI] [PubMed] [Google Scholar]
- Savic S, Bubendorf L. Fluoreszenz-in-situ-Hybridisierung. Eine neue diagnostische Dimension in der Zytologie. [Fluorescence in situ hybridisation. A new diagnostic dimension in cytology.] Pathologe. 2007;28:384–392. doi: 10.1007/s00292-007-0930-x. [DOI] [PubMed] [Google Scholar]
- Ordóñez NG. The diagnostic utility of immunohistochemistry and electron microscopy in distinguishing between peritoneal mesotheliomas and serous carcinomas: a comparative study. Mod Pathol. 2006;19:34–48. doi: 10.1038/modpathol.3800471. [DOI] [PubMed] [Google Scholar]
- Ordóñez NG. Application of mesothelin immunostaining in tumor diagnosis. Am J Surg Pathol. 2003;27:1418–1428. doi: 10.1097/00000478-200308000-00001. (2003b) [DOI] [PubMed] [Google Scholar]
- Kayser K. Personal written communication. June 19, 2006. 2006. (Permission to citation was given by the author to HS on Sept. 4, 2009 in Florence, ESP congress.)
- German Mesothelioma Registry. Mesotheliome. Pictures of three subtypes. Bochum. 2010. http://www.bergmannsheil.de/index.php?id=788&L=1
- Di Loreto C, Puglisi F, Di Lauro V, Damante G, Beltrami CA. TTF-1 protein expression in pleural malignant mesotheliomas and adenocarcinomas of the lung. Cancer Lett. 1998;124:73–78. doi: 10.1016/S0304-3835(97)00466-7. [DOI] [PubMed] [Google Scholar]
- Blobel GA, Moll R, Franke WW, Kayser KW, Gould VE. The intermediate filament cytoskeleton of malignant mesotheliomas and its diagnostic significance. Am J Pathol. 1985;121:235–247. [PMC free article] [PubMed] [Google Scholar]
- Moll R. Cytokeratine als Differenzierungsmarker: Expressionsprofile von Epithelien und epithelialen Tumoren [Cytokeratins as markers of differentiation: Expression profiles of epithelia and epithelial tumours] Stuttgart/Jena/New York, G. Fischer Verlag; 1993. [PubMed] [Google Scholar]
- Suster S, Moran CA. Applications and limitations of immunohistochemistry in the diagnosis of malignant mesothelioma. Adv Anat Pathol. 2006;13:316–329. doi: 10.1097/01.pap.0000213064.05005.64. [DOI] [PubMed] [Google Scholar]
- Winstanley AM, Landon G, Berney D, Minhas S, Fisher C, Parkinson MC. The immunohistochemical profile of malignant mesotheliomas of the tunica vaginalis: a study of 20 cases. Am J Surg Pathol. 2006;30:1–6. doi: 10.1097/01.pas.0000178094.07513.71. [DOI] [PubMed] [Google Scholar]
- Marchevsky AM, Wick MR. Evidence-based guidelines for the utilization of immunostains in diagnostic pathology: pulmonary adenocarcinoma versus mesothelioma. Appl Immunohistochem Mol Morphol. 2007;15:140–144. doi: 10.1097/01.pai.0000213148.62525.9a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of primary and re-evaluation IHC/HC in the 12 cases where MM diagnosis was not confirmed, chronologically.