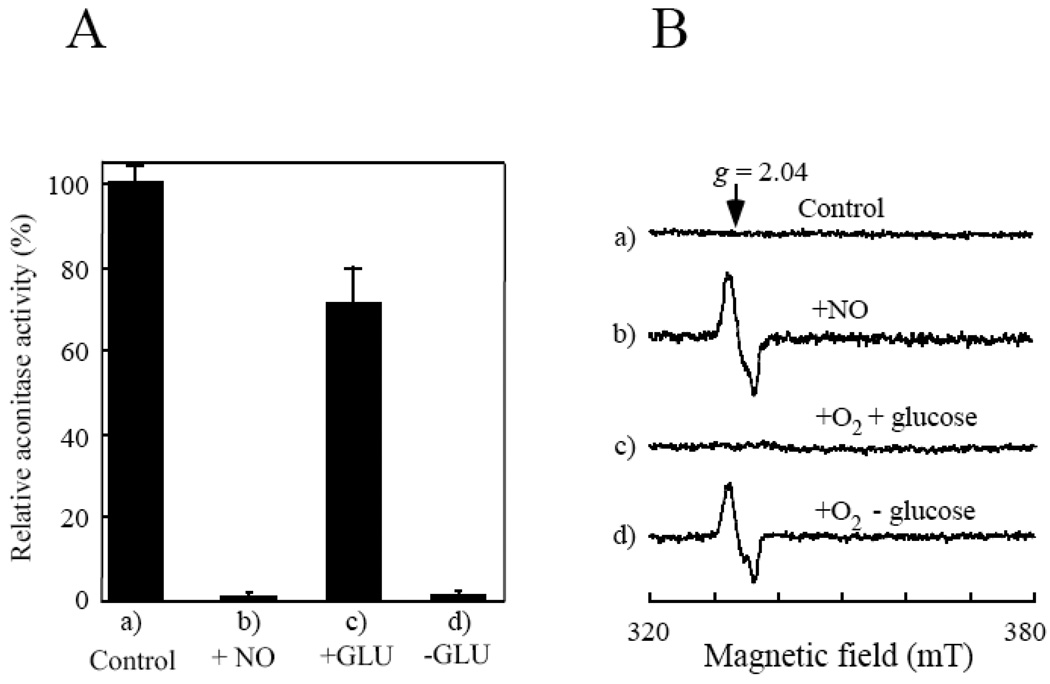
Figure 1. The starved E. coli cells fail to repair the NO-modified iron-sulfur proteins under aerobic conditions.
E. coli cells containing recombinant aconitase B (pBACNB) were washed three times with the M9 minimal medium (without glucose) and re-suspended in the same minimal medium to an OD at 600 nm of 4.0. After 3 hours starvation in the M9 minimal medium (without glucose) under aerobic conditions, chloramphenicol (34 µg/ml) was added to block new protein synthesis before the cells were exposed to NO using the Silastic tubing NO delivery system. A), relative aconitase activity in the cell extracts prepared from a), untreated starved E. coli cells; b), the NO-exposed starved E. coli cells; c), the NO-exposed starved E. coli cells after aerobic incubation in the M9 minimal medium with glucose (0.2%) at 37°C for 60 min; d), the NO-exposed starved E. coli cells after aerobic incubation in the M9 minimal medium without glucose at 37°C for 60 min. The results are averages from three independent experiments. B), the whole cell EPR spectra. The starved E. coli cells (spectrum a) were exposed to NO (spectrum b), followed by re-incubation in the M9 minimal medium with (spectrum c) or without (spectrum d) glucose (0.2%) at 37°C for 60 min under aerobic conditions.