Abstract
Objective: Percutaneous coronary intervention (PCI) triggers an acute inflammatory response, while sirolimus is known to have anti-inflammatory properties; the inflammatory system response to PCI after sirolimus-eluting stent placement remains unclear. The purpose of this study is to determine the changes in high sensitive C-reactive protein (hs-CRP) and apelin after PCI procedure and drug-eluting stent implantation in patients with and without reduced left ventricular systolic function. Methods: Forty-eight consecutive patients undergoing PCI at the Beijing Anzhen Hospital between July and September 2006 were recruited. Sirolimus-eluting stents were employed in all patients. Blood samples were drawn immediately before and 24 h after the procedure. Plasma hs-CRP and apelin levels were determined by enzyme immunoassay. Results: Paired t-test revealed a significant increase in both hs-CRP and apelin post-procedure (P=0.006 and P<0.0001, respectively). Patients with reduced left ventricular ejection fraction (LVEF) had significantly lower baseline apelin levels compared to those with normal ventricular function [(46.8±10.8) vs. (72.0±8.4) pg/ml, P<0.001]. However, apelin increased to a level similar to the level of those with normal left ventricular systolic function 24 h after the PCI procedure [(86.7±11.6) vs. (85.1±6.1) pg/ml, P=0.72]. Conclusions: hs-CRP and apelin levels increased after PCI and sirolimus-eluting stent implantation. Patients with impaired left ventricular systolic function had significantly lower baseline apelin levels, which increased significantly after PCI.
Keywords: High sensitive C-reactive protein (hs-CRP), Apelin, Percutaneous coronary intervention (PCI)
1. Introduction
Each year, millions of patients with coronary heart disease worldwide are treated by means of percutaneous coronary intervention (PCI). Balloon angioplasty and stent implantation can cause endothelial denudation and medial dissection. The inflammatory response to these injuries might be an important mechanism for the initiation of atherosclerosis and restenosis (Kornowski et al., 1998). It has been previously reported that PCI triggers an acute inflammatory response leading to elevations in high sensitive C-reactive protein (hs-CRP) (Almagor et al., 2003), which is implicated in vascular dysfunction and in the progression of atherosclerosis. Sirolimus has been demonstrated to have anti-inflammatory properties (Oberhoff et al., 2002). Whether the inflammatory response to PCI after sirolimus-eluting stent placement is attenuated or not has not been extensively investigated and available data are conflicting (Gogo et al., 2005; Kim et al., 2005; Kochiadakis et al., 2007).
Although application of drug-eluting stents has dramatically reduced the incidence of restenosis after PCI, delayed healing of the traumatized vessel wall and long-term endothelial dysfunction after sirolimus-eluting stent implantation have been reported in porcine and human coronary arteries (Serry and Penny, 2005; Togni et al., 2005; Hofma et al., 2006). Apelin was found recently to be the endogenous ligand of the human orphan receptor APJ (Tatemoto et al., 1998; Szokodi et al., 2002). The restricted presence of apelin in endothelial cells suggests that apelin may play a role as a locally secreted cardiovascular mediator of endothelium function (Kleinz and Davenport, 2004). It has been demonstrated that apelin is decreased in patients with heart failure (Chen et al., 2003). As an endothelium-derived substance, it is not known to what extent apelin will respond to acute coronary artery endothelium damage in PCI patients with and without heart failure. The aim of the present study was to investigate the responses of hs-CRP and apelin to PCI and sirolimus-eluting stent placement in patients with normal and abnormal ventricular function.
2. Patients and methods
2.1. Study population
Consecutive patients with stable or unstable angina pectoris undergoing PCI at the Beijing Anzhen Hospital between July and September 2006 were prospectively recruited. All patients included in the study had a successful procedure defined as a percentage diameter of residual stenosis <50% in the worse of two orthogonal views. All subjects received clopidogrel and aspirin before the procedure. Subjects received enoxaparin 0.5 mg/(kg body weight) intra-arterially at the start of, or enoxaparin 1 mg/(kg body weight) subcutaneously within 6 h before, the procedure. Informed consent was obtained from all participants. Exclusion criteria included PCI within the last 14 d, acute ST-elevation myocardial infarction or cardiogenic shock, presence of infection or inflammatory disease, and contraindication to anticoagulation or excessive risk of bleeding.
2.2. Study protocol
Each subject underwent a physical examination and a structured interview to elicit details of symptoms, past medical history, and medications. The following characteristics were recorded: age, sex, cardiovascular risk factors, acute treatment, and extent of coronary artery disease. Blood samples for serologic analyses were drawn at enrolment from each subject in the sitting position. A 12-lead electrocardiography (ECG) was performed at enrolment. A standardized echocardiogram including a comprehensive 2D, M-mode, and Doppler evaluation was also obtained. Reduced left ventricular ejection fraction (LVEF) was defined as LVEF<40%. Angiography and PCI were performed via the femoral approach using a standard technique. PCI consisted of balloon angioplasty and coronary stenting in all cases. Sirolimus-eluting stents were employed in all subjects. Blood samples for biomarker test were drawn immediately before angiography and 24 h after the procedure.
2.3. Blood sampling and biomarker assay
Blood samples were obtained in ethylenediaminetetraacetic acid (EDTA)-containing tubes and centrifuged. Plasma was extracted and stored at −80 °C until analysis. Plasma apelin levels were determined using a commercially available enzyme immunoassay (Phoenix Pharmaceuticals, Belmont, CA, USA) according to the manufacturer’s instructions. This assay employed an immunoaffinity purified rabbit antibody specific for apelin (Ellinor et al., 2006). Plasma hs-CRP levels were evaluated using a high-sensitivity enzyme-linked immunosorbent assay (ELISA).
2.4. Statistical analysis
Normal distribution for all variables was checked using the one-sample Kolmogorov-Smirnov test. The values were expressed as mean±standard deviation (SD). Differences in the means of the continuous variables were assessed using the two-tailed Student’s t-test. Discrete variables were expressed as rates, and comparisons were tested by χ 2 analysis. Intra-individual comparison between the pre- and post-procedure continuous variables was performed with a paired t-test. A multivariate analysis was performed to determine the correlates of apelin level differences by regressing the difference of apelin levels on clinical variables. Statistical analysis was performed with SPSS 13.0 for Windows (SPSS, Chicago, Illinois, USA). Differences were considered significant with P≤0.05.
3. Results
A total of 48 patients undergoing PCI during the study period were recruited. Twenty-three subjects presented with unstable angina pectoris, and the others had stable angina. Twenty-nine subjects (60.4%) had treated hypertension whilst 11 (22.9%) had a diagnosis of diabetes mellitus. Twelve subjects (25%) had reduced LVEF. All were treated with aspirin and cardioselective β-blockers. Most were on statin treatment prior to PCI. The vessel numbers treated by PCI were 1 in 16 subjects, 2 in 18 subjects, and 3 in 14 subjects. There were no peri-procedure complications, and all had undetectable troponin T levels 24 h after revascularization. Clinical characteristics are presented in Table 1.
Table 1.
Patient characteristics
| Variable | Value* |
| Age (year) | 63.5±10.9 |
| Men | 32 (66.7%) |
| Hypertension | 29 (60.4%) |
| Diabetes | 11 (22.9%) |
| Current smokers | 21 (43.7%) |
| Body mass index (kg/m2) | 25.9±2.8 |
| Total cholesterol (mmol/L) | 4.3±0.8 |
| HDL-cholesterol (mmol/L) | 0.9±0.1 |
| Triglycerides (mmol/L) | 1.8±0.9 |
| Creatinine (mmol/L) | 70.2±17.3 |
| Ejection fraction (%) | |
| Patients with normal LVEF | 64.3±7.6 |
| Patients with reduced LVEF | 34.8±5.1 |
| Systolic blood pressure (mmHg) | 128.6±18.7 |
| Diastolic blood pressure (mmHg) | 75.3±9.4 |
| Medications | |
| Aspirin | 48 (100%) |
| β-blocker | 48 (100%) |
| Statin | 45 (93.7%) |
| Nitroglycerin | 40 (83.3%) |
Values are expressed as mean±SD or n (%)
HDL: high density lipoprotein; LVEF: left ventricular ejection fraction
3.1. hs-CRP levels
hs-CRP increased significantly after the procedure [(1.4±0.9) vs. (4.3±3.5) mg/L, P=0.004]. Paired t-test revealed a significant elevation in hs-CRP levels after PCI compared with baseline. The mean difference between post- and pre-procedure was (2.4±3.6) mg/L, with a test statistic t value of 3.081. The two-tailed probability P value was 0.006.
3.2. Apelin levels
Apelin increased significantly after the procedure [(68.1±13.0) vs. (86.4±10.4) pg/ml, P=0.004]. The difference between post- and pre-procedure was significant when compared by paired t-test. The mean difference was (18.4±14.5) pg/ml with a test statistic t value of 6.21 and the two-tailed probability P value <0.0001.
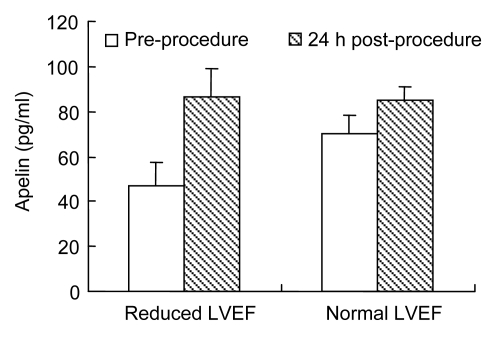
Fig. 1 shows the apelin levels in subjects with normal and reduced LVEF. Apelin levels were significantly lower at baseline in subjects with reduced LVEF [(46.8±10.8) vs. (72.0±8.4) pg/ml, P<0.001], increasing to a level similar to that in those with normal ventricular function 24 h after the procedure [(86.7±11.6) vs. (85.1±6.1) pg/ml, P=0.72]. LVEF was the only variable that independently related to the degree of post-procedure apelin elevation (β=−0.636, standard error (SE)=1.981, P=0.003).
Fig. 1.
Mean plasma apelin levels in subjects with normal and reduced left ventricular ejection fraction (LVEF) pre- and post-procedure
P<0.001 between pre- and post-procedure for each group; P<0.001 between baseline apelin levels; No significant difference (P=0.72) between the two groups after the procedure
4. Discussion
4.1. hs-CRP, PCI, and drug-eluting stents
PCI always induces a systemic inflammatory response and an elevated hs-CRP level. Whether there is a systemic inflammatory response to sirolimus-eluting stent placement is a problem not extensively evaluated to date. In the present study, we demonstrated a three-fold increase in hs-CRP levels after drug-eluting stent placement, an increase similar to reports with bare-mental stents (Gogo et al., 2005).
4.2. Apelin, PCI, and drug-eluting stents
Apelin, the endogenous ligand of the human orphan receptor APJ, was first isolated from the bovine stomach as a 36-amino acid peptide (Tatemoto et al., 1998). APJ receptor, which is closely related to angiotensin II receptor (Habata et al., 1999), is a 380-amino acid seven-trans-membrane domain Gi-coupled receptor that was originally isolated by the polymerase chain reaction (PCR) from human genomic DNA (Hosoya et al., 2000). The biological roles of apelin are only beginning to be explored. Previous work has confirmed that apelin is a powerful vasodilator and positive inotrope and the regulation of apelin system has been studied in the pathological conditions of heart failure (Chen et al., 2003). It has been demonstrated recently that apelin is present to a limited degree in endothelial cells, with a potent ability to stimulate the proliferation of cultured human umbilical vein endothelial cells (Masri et al., 2004).
Endothelial denudation and retarded re-endothelialization after angioplasty injury and drug-eluting stent implantation warn of the increased risk of late stent thrombosis and the impaired endothelial-dependent vasomotion (Bavry et al., 2006). Endothelium-dependent coronary vasomotion adjacent to drug-eluting stents has been demonstrated to be impaired in practically all cases investigated thus far (Serry and Penny, 2005; Togni et al., 2005; Hofma et al., 2006). As the importance of apelin in modulating normal and pathologic angiogenesis is being revealed, apelin up-regulation after angioplasty suggests that it may play a protective pathophysiologic role after endothelium injury. We made the deduction that the apelin system may facilitate the self-renewal of coronary artery epithelium and accelerate structural recovery after injury. Further study on how apelin levels correlate with re-endothelialization status and long-term prognosis is necessary.
It has been demonstrated in animal experiments that apelin infusion acutely improved systolic as well as diastolic left ventricular function (Chen et al., 2003). In fact, apelin is one of the most potent endogenous positive inotropic substances. Recently, low apelin peptide levels were found in failing human hearts, which were up-regulated after the addition of a left ventricular assist device (Chen et al., 2003). We confirmed in the present study that apelin was reduced in subjects with impaired left ventricular function. Furthermore, our study demonstrates that, after PCI, the apelin level increased more prominently in those with reduced LVEF than in those with normal LVEF. Whether the restoration of apelin levels contributes to ventricular function improvement after the procedure of revascularization is yet to be elucidated, and this information will be useful for assessing the role of PCI in the therapy of ischemic heart failure.
Apelin’s physiological effect of lowering blood pressure after intravenous injection into rats had been demonstrated. This effect could be abolished in the presence of a nitric oxide synthesis inhibitor, suggesting that apelin lowers blood pressure through a nitric oxide mechanism (Tatemoto et al., 1998). Apelin may act as a vasorelaxing factor on coronary artery. However, this vasorelaxing effect can only be exerted in the presence of functional endothelium (Kleinz and Davenport, 2004). In endothelium denuded isolated human saphenous veins, apelin is a potent vasoconstrictor, with a maximum response comparable to that of angiotensin II (Katugampola et al., 2001). In the scenario of drug-eluting stent placement, endothelium is denuded and re-endothelialization is retarded. It is not clear whether apelin up-regulation could be a maladaptation that may lead to coronary vasomotion or a protective reaction that may promote endothelium regeneration.
5. Conclusions
hs-CRP levels increased three-fold after PCI and sirolimus-eluting stent implantation despite the anti-inflammatory effects of sirolimus. Apelin levels increased after PCI, especially in patients with impaired left ventricular systolic function. Apelin up-regulation may be an adaptive reaction for the endothelium recovery and ventricular systolic function improvement, while it may be a maladaptation for the vasomotion in the absence of functional endothelium. The pathophysiological effects of apelin with PCI need further study.
Footnotes
Project (No. QN2008-001) supported by the Beijing Municipal Health Bureau Young Persons’ Foundation, China
References
- 1.Almagor M, Keren A, Banai S. Increased C-reactive protein level after coronary stent implantation in patients with stable coronary artery disease. American Heart Journal. 2003;145(2):248–253. doi: 10.1067/mhj.2003.16. [DOI] [PubMed] [Google Scholar]
- 2.Bavry AA, Kumbhani DJ, Helton TJ, Borek PP, Mood GR, Bhatt DL. Late thrombosis of drug-eluting stents: a meta-analysis of randomized clinical trials. The American Journal of Medicine. 2006;119(12):1056–1061. doi: 10.1016/j.amjmed.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 3.Chen MM, Ashley EA, Deng DX, Tsalenko A, Deng A, Tabibiazar R, Ben-Dor A, Fenster B, Yang E, King JY, et al. Novel role for the potent endogenous inotrope apelin in human cardiac dysfunction. Circulation. 2003;108(12):1432–1439. doi: 10.1161/01.CIR.0000091235.94914.75. [DOI] [PubMed] [Google Scholar]
- 4.Ellinor PT, Low AF, MacRae CA. Reduced apelin levels in lone atrial fibrillation. European Heart Journal. 2006;27(2):222–226. doi: 10.1093/eurheartj/ehi648. [DOI] [PubMed] [Google Scholar]
- 5.Gogo PB, Schneider DJ, Watkins MW, Terrien EF, Sobel BE, Dauerman HL. Systemic inflammation after drug-eluting stent placement. Journal of Thrombosis and Thrombolysis. 2005;19(2):87–92. doi: 10.1007/s11239-005-1378-6. [DOI] [PubMed] [Google Scholar]
- 6.Habata Y, Fujii R, Hosoya M, Fukusumi S, Kawamata Y, Hinuma S, Kitada C, Nishizawa N, Murosaki S, Kurokawa T, et al. Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochimica et Biophysica Acta. 1999;1452(1):25–35. doi: 10.1016/S0167-4889(99)00114-7. [DOI] [PubMed] [Google Scholar]
- 7.Hofma SH, van der Giessen WJ, van Dalen BM, Lemos PA, McFadden EP, Sianos G, Ligthart JM, van Essen D, de Feyter PJ, Serruys PW. Indication of long-term endothelial dysfunction after sirolimus-eluting stent implantation. European Heart Journal. 2006;27(2):166–170. doi: 10.1093/eurheartj/ehi571. [DOI] [PubMed] [Google Scholar]
- 8.Hosoya M, Kawamata Y, Fukusumi S, Fujii R, Habata Y, Hinuma S, Kitada C, Honda S, Kurokawa T, Onda H, et al. Molecular and functional characteristics of APJ. Tissue distribution of mRNA and interaction with the endogenous ligand apelin. Journal of Biological Chemistry. 2000;275(28):21061–21067. doi: 10.1074/jbc.M908417199. [DOI] [PubMed] [Google Scholar]
- 9.Katugampola SD, Maguire JJ, Matthewson SR, Davenport AP. [(125)I]-(Pyr(1)) apelin-13 is a novel radioligand for localizing the APJ orphan receptor in human and rat tissues with evidence for a vasoconstrictor role in man. British Journal of Pharmacology. 2001;132(6):1255–1260. doi: 10.1038/sj.bjp.0703939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JY, Ko YG, Shim CY, Park S, Hwang KC, Choi D, Jang Y, Hung N, Shim WH, Cho SY. Comparison of effects of drug-eluting stents versus bare metal stents on plasma C-reactive protein levels. The American Journal of Cardiology. 2005;96(10):1384–1388. doi: 10.1016/j.amjcard.2005.07.042. [DOI] [PubMed] [Google Scholar]
- 11.Kleinz MJ, Davenport AP. Immunocytochemical localization of the endogenous vasoactive peptide apelin to human vascular and endocardial endothelial cells. Regulatory Peptides. 2004;118(3):119–125. doi: 10.1016/j.regpep.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Kochiadakis GE, Marketou ME, Arfanakis DA, Sfiridaki K, Skalidis EI, Igoumenidis NE, Hamilos MI, Kolyvaki S, Chlouverakis G, Kantidaki E, et al. Reduced systemic inflammatory response to implantation of sirolimus-eluting stents in patients with stable coronary artery disease. Atherosclerosis. 2007;194(2):433–438. doi: 10.1016/j.atherosclerosis.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 13.Kornowski R, Hong MK, Tio FO, Bramwell O, Wu H, Leon MB. In-stent restenosis: contributions of inflammatory responses and arterial injury to neointimal hyperplasia. Journal of the American College of Cardiology. 1998;31(1):224–230. doi: 10.1016/S0735-1097(97)00450-6. [DOI] [PubMed] [Google Scholar]
- 14.Masri B, Morin N, Cornu M, Knibiehler B, Audigier Y. Apelin (65–77) activates p70 S6 kinase and is mitogenic for umbilical endothelial cells. The FASEB Journal. 2004;8(5):1909–1911. doi: 10.1096/fj.04-1930fje. [DOI] [PubMed] [Google Scholar]
- 15.Oberhoff M, Herdeg C, Baumbach A, Karsch KR. Stent-based antirestenotic coatings (sirolimus/paclitaxel) Catheterization and Cardiovascular Interventions. 2002;55(3):404–408. doi: 10.1002/ccd.10034. [DOI] [PubMed] [Google Scholar]
- 16.Serry R, Penny WF. Endothelial dysfunction after sirolimus-eluting stent placement. Journal of the American College of Cardiology. 2005;46(2):237–238. doi: 10.1016/j.jacc.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 17.Szokodi I, Tavi P, Foldes G, Voutilainen-Myllyla S, Ilves M, Tokola H, Piuhola J, Rysä J, Tóth M, Ruskoaho H. Apelin, the novel endogenous ligand of the orphan receptor APJ, regulates cardiac contractility. Circulation Research. 2002;91(5):434–440. doi: 10.1161/01.RES.0000033522.37861.69. [DOI] [PubMed] [Google Scholar]
- 18.Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochemical and Biophysical Research Communications. 1998;251(2):471–476. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 19.Togni M, Windecker S, Cocchia R, Wenaweser P, Cook S, Billinger M, Meier B, Hess OM. Sirolimus-eluting stents associated with paradoxic coronary vasoconstriction. Journal of the American College of Cardiology. 2005;46(2):231–236. doi: 10.1016/j.jacc.2005.01.062. [DOI] [PubMed] [Google Scholar]