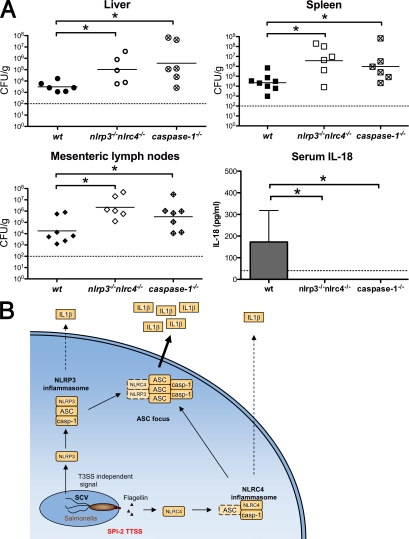
Figure 7.
Redundant roles for NLRP3 and NLRC4 in caspase-1 activation during Stm infection in mice. (A) WT, isogenic caspase-1−/−, and nlrp3−/−nlrc4−/− mice were orally infected with 2.4 × 107 CFU of WT Stm. Liver, spleen, and mesenteric lymph nodes were collected at day 5 after infection. Organ homogenates were diluted and plated to determine cfu per gram of tissue. Bars represent the mean bacterial load. Blood was collected at day 4 after infection and serum IL-18 was determined by ELISA. Data represent the mean ± SD of six to eight mice of each genotype. Results are representative of two independent experiments, each done with groups of six to eight mice of each genotype. Statistical significance was determined using the unpaired Mann-Whitney U test. *, P < 0.05. Dashed lines represent the detection limit of the CFU counts or IL-18 ELISA, respectively. (B) The role of the ASC focus in Stm-induced cytokine processing in vitro. Intracellular Stm activate NLRP3 and NLRC4. NLRC4 responds to flagellin injected by the SPI-2 T3SS, whereas NLRP3 responds to an undefined T3SS-independent signal. Both receptors induce the assembly of inflammasomes that can process pro–IL-1β and pro–IL-18 to their mature forms. NLRP3 inflammasome assembly is completely dependent on ASC, whereas NLRC4 may assemble an inflammasome without ASC. We speculate that ASC then promotes inflammasome aggregation into a single subcellular focus. This ASC focus likely mediates the bulk of cytokine processing and might serve to increase cytokine production in response to continuous stimulation of NLRs. SCV, Salmonella-containing vacuole; casp-1, caspase-1.