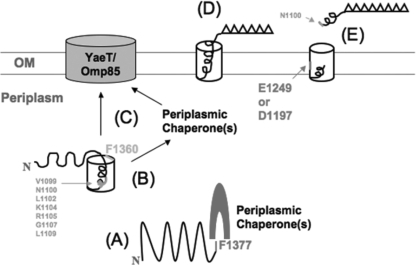
FIG. 6.
Proposed mechanism of the possible roles of the conserved residues located in the translocator domain of a SPATE. Residues shown in a larger font are essential for β-barrel insertion/assembly or passenger processing, as demonstrated in this study; those shown in a smaller font affect secretion, on the basis of the findings of a previous study (18). (A) The folding of the β-domain into a prototertiary structure (12) requires F1377. This process likely involves exogenous secretion factors such as periplasmic chaperones. (B) The folded intermediates are probably stabilized by the formation of the α-helical linker inside the β-barrel. (C) F1360 is required for the proper insertion of the folded translocator domain into the OM, an event also involving exogenous factors such as the Bam complex and, possibly, periplasmic chaperones. (D) Once the translocator domain is properly inserted, the passenger domain folds into its native conformation. (E) E1249 and D1197 then facilitate the cleavage of the passenger domain between N1100 and N1101 in the α-helical linker, resulting in the release of a functional AT into the extracellular environment.