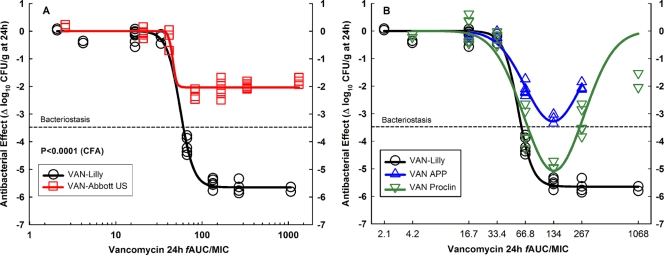
FIG. 1.
In vivo efficacy against S. aureus GRP-0057 (years 2002 and 2003) at a low inoculum (4.30 ± 0.05 log10 CFU per thigh when subcutaneous treatment q1h started). Vancomycin generic products are compared with the innovator (VAN-Lilly) in dose-effect experiments (2.34 to 1,200 mg/kg per day) using the neutropenic mouse thigh infection model (each data point represents the mean CFU/g of both thighs from a single mouse). (A) Pharmacodynamic patterns of VAN-Abbott US and VAN-Lilly fitted to the Hill model. Despite containing a significantly greater concentration of API (125%), VAN-Abbott US was completely ineffective in vivo. VAN-Abbott US is shown in a separate graph because of its greater AUC/MIC ratio than that of VAN-Lilly (123%; their dosing regimens were identical). (B) VAN-APP and VAN-Proclin were both pharmaceutically equivalent to VAN-Lilly, but neither was therapeutically equivalent due to their marked Eagle effect. The curve for VAN-APP ends at 300 mg/kg (fAUC/MIC, 267 h) because this product was discontinued and the remaining amount was insufficient for the highest doses.