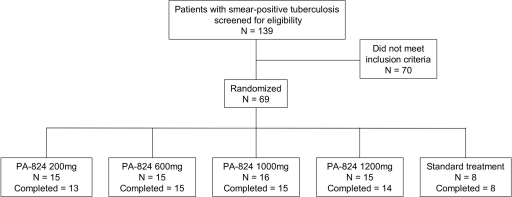
FIG. 1.
Patient disposition. Four participants did not complete the 14-day period of drug intake. Two patients randomized to PA-824 200 mg and one patient randomized to PA-824 1,200 mg were withdrawn due to adverse events not related to study medication (hemoptysis, n = 1; Wolff-Parkinson-White syndrome, n = 1; urinary tract infection, n = 1). One patient randomized to 1,000 mg PA-824 withdrew consent.