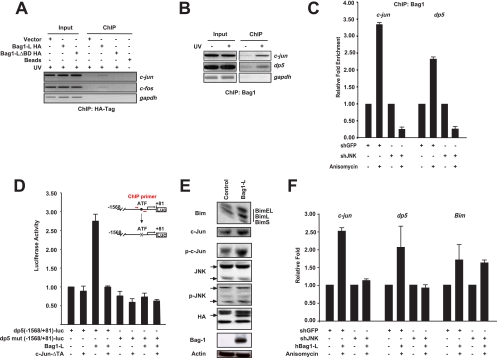
FIG. 5.
Bag1-L positively regulates the proapoptotic markers dp5 and Bim. (A) Chromatin immunoprecipitation was performed using HCT116 cells transfected with empty vector or HA-tagged versions of Bag1-L and Bag1-LΔBD. Cells were subsequently treated with 100 kJ/m2 UV for 2 h prior to collection and processing. ChIP was performed for the HA tag and binding to AP-1 sites in the c-jun and c-fos promoter regions determined by PCR using previously described primers (22, 37). Bag1-L exhibits in vivo occupancy of the c-Jun promoter, and deletion of the Bag domain results in loss of binding to AP-1 elements. (B) Endogenous ChIP was performed using HCT116 cells ± 100 kJ/m2 UV for 2 h prior to cell collection and processing using anti-Bag1. Binding of Bag1 to AP-1 sites in the c-Jun promoter was determined as detailed for panel A, and that to the human c-Jun/ATF-2 [TGATGTAA] site in the dp5 promoter was determined (46). Interaction with AP-1 sites in the c-Jun and dp5 promoters is augmented by JNK activation. (C) HCT116 cells were transfected with the indicated plasmids for 48 h. Cells were treated or not with 25 ng/ml anisomycin for 30 min prior to cell collection and processing. ChIP was performed using anti-Bag1. Binding of Bag1 to AP-1 sites in the c-Jun and dp5 promoters, detailed in panels A and B, was performed by real-time PCR. Enrichment from the IP of each sample was quantified with reference to the individual input, and results are presented as fold enhancement over results for untreated cells and are the means of results for triplicate wells ± SEM. Interaction of Bag1 with AP-1 sites in the c-Jun and dp5 promoters, augmented by anisomycin treatment, is blocked by JNK1/2 knockdown. (D) PC12 cells were differentiated with NGF and transfected to express Bag1-L and/or c-JunΔTA, −1568/+81 dp5-luc (CRE: TGATGTAA) or mut dp5-luc (CRE: TAACGTCT [mutated bases are underlined]), and the Ubi-Renilla-luc transfection control reporter gene (Ubi-Rluc). Luciferase activity was normalized to that for Ubi-Rluc. Data are represented as the means of results for triplicate wells ± SEM. Activity for −1568/+81 dp5-luc + empty vector is arbitrarily set to 1. The ability of Bag1-L to enhance AP-1 reporter activity is blocked by dominant-negative c-Jun. (E) PC12 cells were differentiated with NGF and transfected to express Bag1-L or empty vector. Transfection efficiency was 70%. Cells were collected, extracts were prepared, and immunoblotting was performed with the indicated antibodies. Bag1-L overexpression is accompanied by increased phosphorylated c-Jun levels and the increased expression of the proapoptotic BH3-only protein Bim. (F) HCT116 cells were transfected with the indicated plasmids for 48 h. Cells were treated or not with 25 ng/ml anisomycin for 30 min prior to collection, and mRNA was prepared for quantitative real-time-PCR. Results were normalized to those obtained with gapdh, and results are presented as fold induction over that for untreated cells and are the means of data for triplicate wells ± SEM. Primer sequences used were as previously described for c-Jun (42), dp5 (35), and Bim (7). The increase in c-Jun and dp5 transcript levels induced by anisomycin treatment is blocked by shJNK, but that of Bim is unaffected.