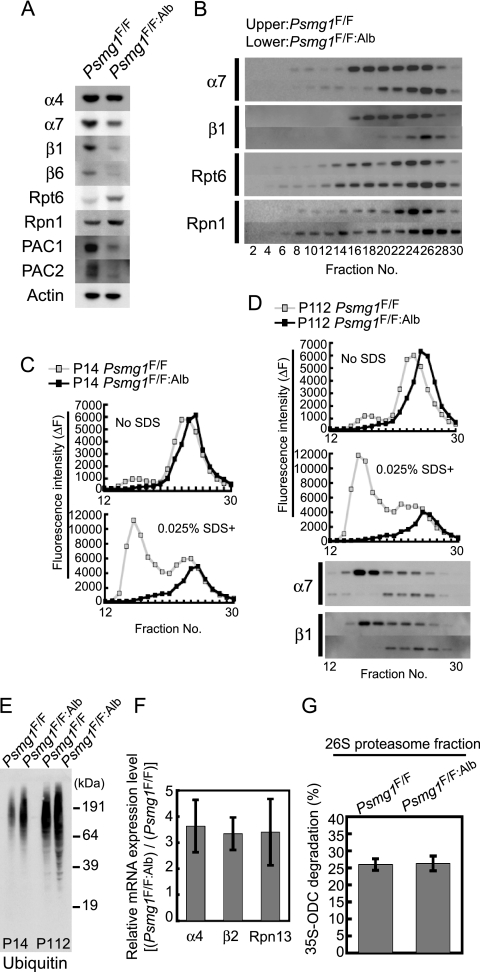
FIG. 4.
Loss of latent 20S proteasomes causes inefficient degradation of ubiquitinated proteins. (A) Immunoblot analysis of liver extracts from Psmg1F/F and Psmg1F/F:Alb mice at P14 using the indicated antibodies. (B) The extracts shown in panel A were fractionated by 8 to 32% glycerol gradient centrifugation and subjected to immunoblot analysis using the indicated antibodies. (C) The fractions shown in panel B were subjected to an assay of the chymotryptic activity of the proteasome using Suc-LLVY-MCA in the absence (upper panel) or presence (lower panel) of 0.025% SDS. (D) Liver extracts from Psmg1F/F and Psmg1F/F:Alb mice at P112 were analyzed as described for panels A and B. (E) Immunoblot analysis of liver extracts from Psmg1F/F and Psmg1F/F:Alb mice at P14 and P112 using antiubiquitin antibody. (F) Transcription levels of proteasome subunits in P14 liver analyzed by real-time PCR. The relative ratios of the expression levels in Psmg1F/F:Alb liver compared to those in Psmg1F/F liver are shown. The data represent means ± standard deviations (SD) from three independent experiments. (G) Fraction 26 shown in panel B was subjected to an S35-ODC degradation assay. The data represent means ± SD from five independent experiments.