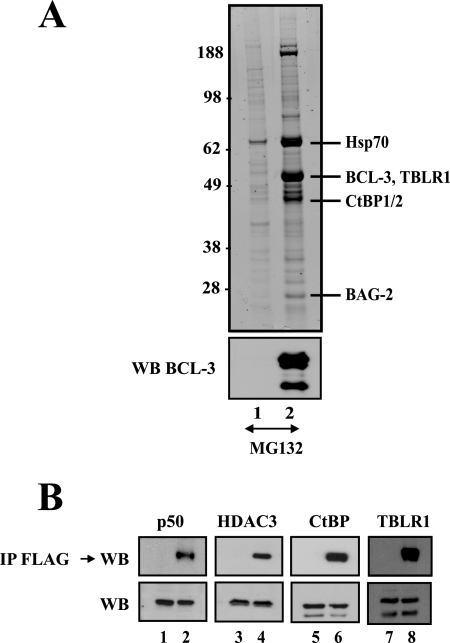
FIG. 1.
Identification of multiple BCL-3-associated proteins through biochemical purification. (A) (Top) Mock-expressing 293 cells or 293 cells stably expressing FLAG-BCL-3, treated with MG132 (20 μM) for 4 h, were subjected to anti-FLAG immunoprecipitation (lanes 1 and 2, respectively). Immunoprecipitated proteins were subsequently released from the beads using the FLAG peptide and were subjected to SDS-PAGE, silver staining, and mass spectrometric analysis for identification. (Bottom) Western blotting (WB) with an antibody against BCL-3 was carried out on the immunoprecipitates. (B) (Top) Western blot analysis of anti-FLAG immunoprecipitates (IP) from MG132-treated mock-expressing or FLAG-BCL-3-expressing cells (odd and even lanes, respectively). Western blotting was carried out with antibodies against p50 and HDAC3 (positive controls) and against CtBP and TBLR1 (identified by mass spectrometry), as indicated, to validate the identifications obtained by mass spectrometry. (Bottom) Western blotting was also carried out with antibodies against p50, HDAC3, CtBP, and TBLR1 by using 5% of the extracts used in immunoprecipitation experiments.