FIG. 4.
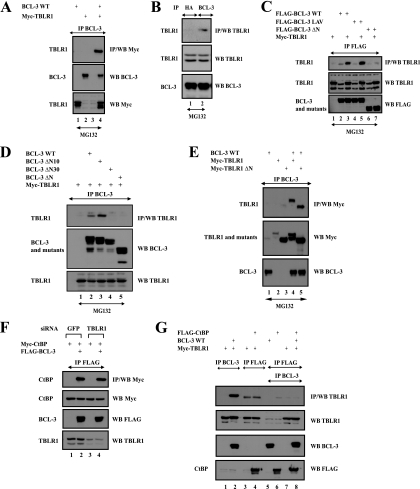
TBLR1 interacts with BCL-3 through the N-terminal domain but not with CtBP. (A and B) BCL-3 and TBLR1 interact in transfected 293 cells and at the endogenous level. (Top) Cell extracts from 293 (A) or Karpas (B) cells treated with MG132 (20 μM) were subjected to immunoprecipitation (IP) with an anti-HA (B) (negative control) or anti-BCL-3 antibody, followed by Western blot analysis (WB) with an anti-Myc (A) or anti-TBLR1 (B) antibody. (Center and bottom) Crude cell extracts were subjected to Western blotting with antibodies against TBLR1 (B), Myc (A), and BCL-3 (A and B) as well. (C and D) BCL-3 associates with TBLR1 through its N-terminal domain. 293 cells were transfected with the indicated expression plasmids, and cell extracts were subjected to immunoprecipitation and Western blot analysis, as indicated. (E) The N-terminal LisH domain of TBLR1 is dispensable for binding to BCL-3. (Top) 293 cells were transfected with the indicated expression plasmids, and cell extracts were subjected to immunoprecipitation with an anti-BCL-3 antibody, followed by Western blotting with an anti-Myc antibody. (Center and bottom) Cell extracts were also subjected to Western blotting with antibodies against Myc and BCL-3. (F) TBLR1 is dispensable for the association of BCL-3 with CtBP. (Top panel) 293 cells depleted of GFP (negative control) or TBLR1 were transfected with the indicated expression plasmids, and cell extracts were subjected to immunoprecipitation with an anti-FLAG antibody, followed by Western blotting with an anti-Myc antibody. (Center and bottom panels) Cell extracts were subjected to Western blotting with antibodies against Myc, FLAG, and TBLR1 as well. (G) Evidence for distinct CtBP- and TBLR1-containing BCL-3 subcomplexes. (Top panel) 293 cells were transfected with the indicated expression plasmids, and cell lysates were subjected to immunoprecipitation with an anti-BCL-3 (lanes 1 and 2) or anti-FLAG (lanes 3 to 8) antibody. The resulting immunoprecipitates were subsequently subjected to Western blotting with an anti-TBLR1 antibody (lanes 1 to 4) or were released from the beads using a FLAG peptide (lanes 5 to 8). The released materials were subsequently subjected to immunoprecipitation with an anti-BCL-3 antibody followed by Western blotting with an anti-TBLR1 antibody (lanes 5 to 8). (Center and bottom panels) Crude cell extracts were subjected to Western blotting with antibodies against TBLR1, BCL-3, and FLAG as well.