Abstract
In order to meet planetary-protection requirements, culturable bacterial spore loads are measured representatively for the total microbial contamination of spacecraft. However, the National Aeronautics and Space Administration's (NASA's) cotton swab protocols for spore load determination have not changed for decades. To determine whether a more efficient alternative was available, a novel swab was evaluated for recovery of different Bacillus atrophaeus spore concentrations on stainless steel and other surfaces. Two protocols for the nylon-flocked swab (NFS) were validated and compared to the present NASA standard protocol. The results indicate that the novel swab protocols recover 3- to 4-fold more (45.4% and 49.0% recovery efficiency) B. atrophaeus spores than the NASA standard method (13.2%). Moreover, the nylon-flocked-swab protocols were superior in recovery efficiency for spores of seven different Bacillus species, including Bacillus anthracis Sterne (recovery efficiency, 20%). The recovery efficiencies for B. atrophaeus spores from different surfaces showed a variation from 5.9 to 62.0%, depending on the roughness of the surface analyzed. Direct inoculation of the swab resulted in a recovery rate of about 80%, consistent with the results of scanning electron micrographs that allowed detailed comparisons of the two swab types. The results of this investigation will significantly contribute to the cleanliness control of future life detection missions and will provide significant improvement in detection of B. anthracis contamination for law enforcement and security efforts.
The recent discovery of liquid water on Mars has sparked debate about the possibility of extraterrestrial life (37). Consequently, highly sensitive biosensors will be deployed onboard spacecraft like the Mars Science Laboratory (MSL), using technologies such as gas chromatographical analysis to search for the smallest traces of life (http://mars.jpl.nasa.gov/msl/mission/). Contamination of equipment by terrestrial microorganisms resulting from a lack of spacecraft cleanliness could significantly compromise the integrity of life detection missions and result in falsely positive extraterrestrial life signals. The prevention of this so-called “forward contamination” is one major goal of American and European space agencies' planetary-protection efforts. Regular determination of a spacecraft's bioload and the mission components throughout assembly are mandatory for detecting unacceptably high contamination that exceeds levels set by the United Nations treaty (Outer Space Treaty [11]).
Modern spacecraft hardware is very susceptible to standard heat sterilization protocols, so baking the entire spacecraft, such as the Viking Lander Capsule at 111.7°C ± 1.7°C for 23 to 30 h is no longer feasible (30). Alternative cleaning and sterilization methodologies for spacecraft components prior to assembly (i.e., nonthermal plasma technologies) have been discussed (36). However, after integration, sterile hardware is exposed to a significant risk of contamination during assembly, testing, and launching operations. Because of limited access to integrated spacecraft components, the microbial cleanliness of a spacecraft and its surroundings is meticulously maintained through frequent cleaning and sterilization routines. Therefore, the regular and frequent detection of possible contaminants in the assembly environment is more important than ever.
To estimate the severity of microbial contamination, the National Aeronautics and Space Administration's (NASA's) standard procedure focuses on aerobic, mesophilic spores (26). Briefly, surface samples are taken from spacecraft using moist cotton swabs or wipes. After an extraction procedure, the samples are subjected to a short heat shock (15 min; 80°C) to kill vegetative cells and then pour plated in Trypticase soy agar (TSA) for the enumeration of CFU. This protocol was originally developed for the Viking mission more than 3 decades ago (30) and has remained, for the most part, unchanged.
Recent studies have shown that cotton swabs have acceptable recovery efficiencies for Bacillus spores (41.7%) (32) but, due to their organic nature, may raise residue problems on surfaces. Furthermore, their comparatively high DNA content could lead to false positives or inhibition should NASA one day incorporate molecular technologies into their microbial-detection protocols (7).
Based on these observations, researchers are beginning to move away from cotton in favor of alternative swabs made from rayon or macrofoam (6, 18). A recent study reported high recovery efficiencies for various vegetative cells from stainless steel surfaces by applying a novel swab with a bulb-shaped head flocked with nylon fibers (12). Patented in 2004, this design facilitates the release of particulates and microbes, resulting in a significantly higher detection rate. The broad applicability of these nylon-flocked swabs (NFS) has been demonstrated by their use in various clinical studies isolating pathogens from medical environments (1, 10, 20).
General studies on surface-sampling tools have clearly shown that the swab material and the extraction method are the dominant factors in spore recovery efficiencies (32). Additionally, the properties of the surface to be sampled affect sample recovery (8). For planetary-protection applications, the broad variety of novel materials used in spacecraft construction must be considered. The Mars Exploration Rover mission craft, for example, was composed of at least five kinds of surface materials (http://marsrovers.jpl.nasa.gov/overview). While the cruise stage was constructed primarily of aluminum and the aeroshell consisted of aluminum honeycomb structures, the lander itself was made of titanium and graphite composite (carbon fiber-reinforced plastic [CFRP]). The airbag and the parachutes were made of Vectran and polyester/nylon fabrics. These different materials are quite challenging for sampling tools. Accurate sampling of materials with various surface textures will require planetary-protection programs to introduce novel swab materials.
To our knowledge, no investigations have been performed to compare the recovery of spores from different spacecraft surfaces. Previous studies have compared cotton and synthetic sampling materials, but only on stainless steel surfaces (19), and no studies have compared sampling methods on actual spacecraft materials (7).
Recently published protocols for spore detection have been based on one specific Bacillus species and/or on one type of surface. Unfortunately, these protocols provide no insight into the effects of varying these factors (4-6, 8, 9, 14, 18), as requested by USP (United States Pharmacopeia) 1223 for validation of alternative microbial methods (3). Some of the aforementioned studies were conducted in response to B. anthracis terrorism incidents in 2001 and used B. atrophaeus as a surrogate. Consequently, information about the actual sampling efficiency of B. anthracis spores is quite limited and may vary significantly from the B. atrophaeus data.
In this comprehensive study, we evaluated the novel nylon-flocked swab and a corresponding protocol to recover Bacillus spores from five different spacecraft-related surfaces. It should be noted that although stainless steel served as the standard test surface, it is not a predominant material in spacecraft; however, since the majority of previous (sampling) studies were performed on stainless steel, it represents a universally recognized carrier and also serves as a conservative proxy for the average roughness of the materials used in space science.
Our nylon-flocked-swab protocol was validated with respect to accuracy, precision, limit of detection, linearity, and robustness (3). Moreover, its specificity was determined by applying spores of seven different Bacillus species, including the avirulent, attenuated strain Bacillus anthracis Sterne, and by comparing the resulting recovery efficiencies. The results in this communication will significantly contribute to planetary-protection protocols and could also be of high interest for public health issues.
MATERIALS AND METHODS
Experimental design and general background.
Focusing primarily on planetary-protection questions, the NFS was validated in a broad variety of experiments. The novel swab was tested in two sampling, extraction, and culturing protocols with different spore concentrations and seven different Bacillus species and on five different surfaces in order to obtain a more complete understanding of its capabilities. The purpose of this work was to evaluate an alternative method that simultaneously provided improved usability (e.g., spread instead of pour plating) and superior detection of spores, based on the present NASA standard procedure. A comparison of the current NASA method and the chosen NFS protocols A and B is provided in Table 1. The maximum spore concentration to be tested was set at 100 CFU per 25 cm2, corresponding to 4 × 104 CFU per m2. This (maximum) cultivable cell concentration was based upon previous measurements of microbial clean-room contamination, based on attempts at cultivation of heterotrophic bacteria (22). The general guideline for the evaluation of the NFS protocols was USP 1223 (3). Among the criteria listed in the USP guidelines, the accuracy, precision (repeatability), specificity (applicability to different types of spores), linearity (applicability to different [low] spore concentrations), and robustness (reliability under variations of the procedure) of the novel NFS protocols were of major interest.
TABLE 1.
Comparison of the current NASA standard procedure for the detection of contamination on spacecraft and alternative methods using nylon-flocked swabs
| Parameter | Value for: |
Purpose of modification | ||
|---|---|---|---|---|
| NASA standard | Protocol A | Protocol B | ||
| Swab | Cotton swab | Nylon-flocked swab | Nylon-flocked swab | Cotton swab causes residues on surfaces and might inhibit/contaminate molecular analyses |
| Storage solution | Water | PBST | Water | For the detection of vegetative cells, PBST was tested as an alternative |
| Vol (ml) | 10.0 | 2.5 | 2.5 | The volume was decreased to improve handling and reduce risk of losses |
| Extractiona | Sonication and vortexing | Vortexing | Sonication and vortexing | Vortexing is preferred as a sole extraction method for vegetative cells |
| Medium | TSA | R2A | R2A | R2A is more suitable for the cultivation of microbes adapted to low organic concentrations |
| Plating technique | Pour plating | Spread plating | Spread plating | Spread plating facilitates on-site contamination control |
Vortexing, 5 to 6 s; sonication, 120 s.
NFS protocols A and B, meanwhile, were included in the European Space Agency's (ESA's) standard (15).
Bacillus species.
Overall, seven different Bacillus species were used for this study, while B. atrophaeus DSM 675T served as the standard test species. B. anthracis Sterne 34F2 was provided by U. Reischl, Institut fuer Medizinische Mikrobiologie und Hygiene, Universitaetsklinik, Regensburg, Germany; the two strains B. megaterium 2c1 and B. thuringensis E24 were original isolates from spacecraft assembly clean-room environments and were provided by P. Rettberg, DLR, Cologne, Germany. All other species were type strains, available from DSMZ (Deutsche Sammlung von Mikroorganismen und Zellkulturen), B. safensis DSM 19292T, B. megaterium DSM 32T, and B. thuringensis DSM 2046T).
Spore stock solutions.
Spores were isolated as described elsewhere (24). In brief, spores were isolated via lysozyme and DNase treatments. Enzymes were inactivated by heat shock (15 min; 80°C). The spores were washed with H2O and then stored in 50% (vol/vol) ethanol (EtOH) to avoid germination. For stock solutions, spores were diluted to a concentration of 100 CFU per 200 μl solution as confirmed by spread plating them on R2A (BD, Heidelberg, Germany). Incubation was performed at 32°C, and colonies were counted after 24, 48, and 72 h.
Incubation medium tests.
B. atrophaeus spores (100 CFU) were plated in parallel on R2A and TSA plates (10 replicates). Incubation was performed at 32°C, and colonies were counted after 24, 48, and 72 h.
Sample surface material.
All surfaces were provided in a size of 5 × 5 cm, if not otherwise stated. Besides stainless steel, the following materials were linked to ESA's present ExoMars mission and therefore considered for analysis. (i) Stainless steel coupons (Wilms' Metallmarkt Lochbleche, Cologne, Germany), punched out from stainless steel (1.4301), grounded (240 grain, foil; 1.5 mm by 50.5 mm by 50.5 mm). This surface served as a reference for the basic evaluation of the swab protocol. (ii) Laminated and cured carbon fiber-reinforced plastic (HexPly 913; Hexcel). (iii) Laminated and cured carbon-reinforced plastic (HexPly 913; Hexcel) roughened by applying Release Ply reinforcement (commonly used prior to gluing or varnishing the carbon fiber). (iv) Vectran fabric type A, provided by AeroSekur, Aprilia, Italy. The Vectran yarns (220 decitex = 200 denier) were twisted, and the fabric contained 24 ± 1 yarns/cm. On the rear, the fabric had been coated with Elastosil LR 3003/30 A/B liquid silicone rubber (Wacker, Burghausen, Germany). The total weight of the fabric was 201 g/m2 (±15%; without coating, 135 g/m2). (v) Vectran fibers, type B, also provided by AeroSekur. The Vectran yarns (280 decitex = 250 denier) were twisted, and the fabric contained 24 ± 1 yarns/cm. The fabric had been coated with silicon (LR 3003 A&B; black) on the rear. The final weight of the fabric was 200 g/m2 (±15%; without coating, 145 g/m2).
Surface preparation prior to sampling.
All surface materials were rinsed with 70% (vol/vol) EtOH and sterilized as follows. Stainless steel coupons were sterilized at 160°C for 3 h (dry heat). Alternate surfaces were sterilized via UV irradiation (GS Gene Linker, UV chamber; Bio-Rad) and autoclaving (121°C; 200 kPa; 1 h). For inoculation, spores were spotted (small drops, ∼4 μl) onto the corresponding surface. The test surfaces were dried under the laminar flow for 24 h at room temperature with no influence on spore viability (34).
Swab description.
The NFS (552C regular swab; ethylene oxide [EO] sterilized; Copan, Brescia, Italy) consisted of nylon fibers attached to the head of the swab. In the middle of the shaft, a predetermined breaking point ensured easy separation of the head from the handhold after sampling. Further on, each swab was aseptically packed in separate, sealed containers. For comparative studies, cotton swabs (150C; radiation sterilized; wooden stick; Copan) were used. These swabs were also packed in sterile containers but had no predetermined breaking points. The NASA standard protocol does not specify the supplier of cotton swabs with wooden sticks to be used and describes the preparation and sterilization procedure for nonpresterilized swabs. For this study, cotton swabs were chosen that were available already presterilized and prepacked in single containers to ensure the same quality for all tests.
General handling and procedures.
All experiments described here were carried out under suitable cleanliness conditions of a laminar flow (Microflow Biological Safety Cabinet; Nunc, Wiesbaden, Germany). For measuring the microbiological contamination level based on the aerobic, mesophilic culturable-spore number, the space agency's standard procedures contain a heat shock step (15 min; 80°C) within the extraction procedure in order to kill all vegetative cells (15, 26). This step was omitted in this study, since only Bacillus spores were used for the experiments.
Nylon-flocked-swab protocol A.
First, a sterile dry swab was aseptically removed from its container, and the head of the swab was moistened in a test tube containing 1 ml of sterile water. Excess moisture was removed from the swab by squeezing the swab against the interior wall of the tube. The swabbing was done by holding the swab at a 30° angle toward the surface to be sampled. While the swab was moved in one direction, the head of the swab was rotated slowly and thoroughly over the surface. The linear direction of the swabbing motion was changed by 90°, and the surface was again swabbed thoroughly. A third coverage of the surface was completed by changing the direction of the swabbing motion by 135°. The swab head was returned to a sterile tube containing 2.5 ml sterile buffer (phosphate-buffered saline [PBS] including 0.02% [vol/vol] Tween 80 [PBST]) by breaking the swab shaft at the breaking point. In the experiment, the tube containing the swab head was stored at room temperature (RT; 22°C ± 2°C) for 1 h in order to simulate transportation from the sampling location to the laboratory. For sample processing, the tube containing the swab head was vortexed (Genie 2;, Scientific Industries, Bohemia, NY) at full speed for 5 to 6 s directly prior to plating. Then, four 0.5-ml samples of the spore suspensions were plated on agar plates (R2A) using sterile disposable spreaders. For lower spore numbers, two 1-ml samples of the spore suspensions were used. The plates were incubated at 32°C as stated in the ESA standard protocol (15). Colony counts were done after 24, 48, and 72 h. Negative controls were carried through all tests, that is, sterile 50% (vol/vol) EtOH was spotted on one sample surface, followed by the swab protocol described above.
Nylon-flocked-swab protocol B.
To compare two different methods for spore storage and extraction, the protocol mentioned above was modified as follows. After sampling, the swab was broken into a test tube containing 2.5 ml sterile, distilled water and stored at RT for 1 h. Then, the spores were extracted by vortexing (5 to 6 s) followed by sonication (84 W; 35 kHz; Sonorex super DK 102P ultrasonic device; Bandelin, Germany) for 120 s. Samples were processed according to protocol A, as described above.
NASA standard swab assay.
For evaluation of the NFS protocols, the method was compared to the NASA standard method (26). In brief, this assay is based on the following steps. First, the swab was moistened with sterile water, prior to swabbing the test surface three times as outlined for NFS protocol A. The head of the swab was then returned, by cutting off the head, to the original tube of solution, containing 10 ml of distilled, sterile water. After incubation at RT for 1 h, the tube containing the swab head was vortexed for 5 to 6 s and sonicated for 120 s. For pour plating, two 4.0-ml portions of extraction suspension were mixed with 20 ml of sterile, molten (48 to 50°C) TSA. The solid plates were incubated at 32°C. Colony counts were done after 24, 48, and 72 h.
Direct inoculation.
For determination of the extraction efficiency, spores of B. atrophaeus (100 CFU) were directly spotted onto the swab head. Then, the swab was broken into 2.5 ml sterile water, followed by extraction and CFU enumeration as described above for NFS protocol B. For statistical analysis (see below), the volume was adjusted to 2.7 ml (2.5 ml storage solution and 0.2 ml spore solution).
Worker variability.
For comparative purposes, one test series based on protocol A was repeated by an experimenter who had not performed swabbing of surfaces before. This was done to test the reliability and robustness of the method.
Statistical analysis.
In adherence to the stipulations of USP 1223 (3), the statistical processing of the data made proper use of the underlying probability distribution of the raw data, i.e., the Poisson distribution, instead of approximations to the normal distribution. In particular, two features of Poisson-distributed data were exploited in the analysis (27, 29, 33). Given count data, x and y, from two Poisson populations with expectation values (means) ξ and η, i.e., X∼Poisson(ξ) and Y∼Poisson(η), then the sum, s = x + y, is again a Poisson variable, S∼Poisson(ζ), with mean ζ = ξ + η. This is generalizable to more than two samples.
The test of equality of two means or the construction of confidence intervals for an observed ratio r = x/y is based on a second property of the Poisson distribution. Given the above observed sum, s = x + y, the distribution of Y or X conditional on S = s is a binomial distribution,
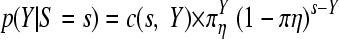 |
(1a) |
or
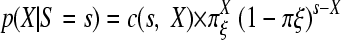 |
(1b) |
with πη = η/(η + ξ), πξ = ξ/(η + ξ), and the combinatorial coefficient c(n, k) = n!/(k! (n − k)!).
The null hypothesis ξ = η translates to πη = πξ = 1/2.
In cases where x = Σxi, i = 1 … M, Xi∼Poisson(ξ), and y = Σyj, j = 1 … N, Yj∼Poisson(η), πη = Nη/(Nη + Mξ), and πξ = Mξ/(Nη + Mξ).
Now the null hypothesis ξ = η is represented by πη = N/(N + M) or πξ = M/(N + M).
The 1-sided type I error probabilities, α(1), for the null hypothesis ξ = η are obtained by summing p(Y | S = s) and/or p(X | S = s), depending on the envisaged deviation (less than or more than) under the alternative hypotheses.
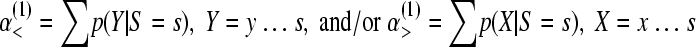 |
(1c) |
Confidence intervals for ratios, R, estimating ρ = ξ/η from observations of counts u and v, U∼Poisson(aξ) and V∼Poisson(bη) with u + v = w and known a and b again are derived from the binomial distribution.
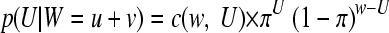 |
(2a) |
with π = aξ/(aξ + bη) = a ρ/(a ρ + b), whence ρ = b π/(a (1 − π)).
With the estimate for π, p = u/(u + v), and the lower and upper binomial confidence limits pl, pu derived thereby, the confidence limits for the estimate
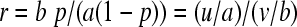 |
(2b) |
are obtained from
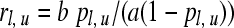 |
(2c) |
Data were processed by a program script for the statistical programming environment R (http://www.R-project.org). In particular, the R functions pois.exact(), binom.exact(), and pbinom() were used for calculations according to equations 1 and 2. Confidence levels were chosen as 0.95 (95%). Statistical-analysis results were visualized using Microsoft Excel and OriginPro version 7.5 (OriginLab Corporation), respectively.
For the three recovery methods, average recoveries across the test species or across the test inocula were determined as weighted averages. The inverse squares of the relative widths of the confidence intervals were taken as weights. The uncertainty of these averages is specified by the corresponding standard error of the mean. For application of such data in probabilistic assessments of microbial clean-room contaminations, instead of the standard error, the standard deviation is required, which is also given.
Calculation of the number of replicates.
The number of replicates was chosen based on the accepted measurement inaccuracy of 10% and considering a recovery efficiency of 50%. Considering the Poisson distribution, the variance of a counting result, V(N) = N. The standard deviation S is a measure of the measurement precision: S = 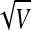 =
= 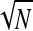 . The relative measurement precision, ζ = S/N, is therefore ζ = 1/
. The relative measurement precision, ζ = S/N, is therefore ζ = 1/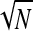 (N, counts). The number of replicates for an experiment as given in Table 2 was chosen by aiming at an approximately equal ζ, depending on the different expected total number, NT, of recovered CFU. Pilot experiments for each of the protocols yielded this expected total number.
(N, counts). The number of replicates for an experiment as given in Table 2 was chosen by aiming at an approximately equal ζ, depending on the different expected total number, NT, of recovered CFU. Pilot experiments for each of the protocols yielded this expected total number.
TABLE 2.
Linearity of the nylon-flocked-swab protocol compared to the NASA standard protocola
| Recovery method | Spore inoculum (CFU nominal/actual) | No. of replicates | Mean recovery (CFU) | Range of recovery (CFU) | Recovery efficiency (%)b | 95% CIc | Avg recovery (%)d |
|---|---|---|---|---|---|---|---|
| Nylon-flocked-swab protocol Ae | 100/100.5 | 10 | 45.5 | 35.0-55.0 | 45.2 | 40.2-50.7 | 45.4 ± 1.2 (2.74) |
| 15/15.4 | 20 | 6.3 | 1.2-11.1 | 41.1 | 31.8-52.9 | ||
| 10/10.4 | 30 | 5.1 | 1.2-10.0 | 49.1 | 37.7-63.9 | ||
| 5/5.1 | 40 | 2.4 | 0.0-6.3 | 46.9 | 33.9-65.0 | ||
| 3/3.25 | 50 | 1.5 | 0.0-7.5 | 47.3 | 33.5-66.7 | ||
| Nylon-flocked-swab protocol Be | 100/99.1 | 10 | 50.6 | 36.2-61.2 | 51.1 | 45.9-56.8 | 49.0 ± 1.9 (4.18) |
| 15/15.3 | 20 | 7.7 | 1.2-18.6 | 50.1 | 40.2-62.2 | ||
| 10/10.5 | 30 | 4.5 | 1.3-8.8 | 43.3 | 34.4-54.2 | ||
| 5/5.4 | 40 | 2.3 | 0.0-6.1 | 42.8 | 31.9-57.0 | ||
| 3/3.3 | 50 | 1.5 | 0.0-5.0 | 46.0 | 33.0-63.5 | ||
| Cotton swab, NASA standardf | 100/99.1 | 10 | 14.3 | 11.3-17.6 | 14.4 | 11.8-17.4 | 13.2 ± 1.2 (2.32) |
| 15/15.3 | 20 | 1.4 | 0.0-4.7 | 9.4 | 5.8-14.4 | ||
| 10/10.5 | 30 | 1.3 | 0.0-3.9 | 11.9 | 7.9-17.4 | ||
| 5/5.4 | 40 | 0.7 | 0.0-2.8 | 12.1 | 7.3-19.3 | ||
| Nylon-flocked swab, NASA standard | 100/100.5 | 10 | 30.0 | 15.0-47.5 | 29.9 | 26.0-34.2 | ND |
| Nylon-flocked swab, worker variabilityg | 100/100.5 | 10 | 31.6 | 23.8-55.0 | 39.3 | 34.7-44.4 | ND |
Surface, stainless steel coupons; spores, B. atrophaeus.
Recovery efficiency calculated from the mean number of recovered spores.
CI, confidence interval of recovery efficiency.
±Standard error of the mean (standard deviation). For further details, see Materials and Methods. ND, not determined.
Protocol A was based on PBST for storage and vortexing for extraction; protocol B was based on water for storage and vortexing and sonification for extraction.
No test series for the NASA standard method was accomplished with 3 CFU per coupon, as this concentration was below its LOD.
Inexperienced worker who had not done swabbing before; the experiment was performed according to protocol A.
SEM and spore purification.
For scanning electron microscopy (SEM), B. atrophaeus spores that were obtained according to the method of Moeller et al. (24) were in need of further purification to remove organic mother cell remnants still present in the spore solution. Therefore, ultracentrifugation was carried out using a CsCl gradient (40% [wt/wt]) (swing-out rotor SW 60 Ti; Beckman Optima LE-80K; Beckman Coulter Inc., CA) as described previously for spore crystal purification of B. thuringiensis (16). After centrifugation (16 h; 50,000 rpm [336,239 × g]), visible bands were removed using syringes with hypodermic needles and analyzed by phase-contrast microscopy. Fractions containing purified spores were diluted with the same amount of pure water and then washed three times with pure water (13,000 rpm [13,600 × g]; 10 min) (Biofuge 13; rotor no. 3757; Heraeus, Hanau, Germany). The spores were then stored in sterile 50% (vol/vol) EtOH for further analysis. To visualize different steps of the nylon-flocked-swab protocol, 108 highly purified spores of B. atrophaeus were spotted on stainless steel coupons and dried for 24 h as described above. Sampling was performed according to the protocol, and the heads of the swabs were removed directly after sampling and after extraction. These preparations were analyzed by scanning electron microscopy, which was carried out using a Digital Scanning Microscope (DSM 950; Zeiss, Oberkochen, Germany). Prior to this, preparations were coated with a gold/palladium target, creating a layer of 1.4 nm (Polaron SC 515, SEM Coating System).
RESULTS
Incubation medium tests.
B. atrophaeus spore solutions were plated in parallel on TSA and R2A, resulting in no detectable difference in the numbers of CFU (data not shown). Therefore, R2A was used for all further experiments, as it is also specified in the ESA standard protocol (15).
Comparison of methods.
B. atrophaeus spores (100 CFU) were spotted on stainless steel coupons and sampled by one of the three described methods. As shown in Table 2, protocol A resulted in a recovery efficiency of 45.2% and protocol B 51.1%, whereas application of the NASA standard protocol revealed an efficiency of only 14.4%. Based on these results, both NFS protocols were superior to the NASA standard procedure for B. atrophaeus spore recovery.
In an additional test, the NASA standard protocol was used to sample a nominal 100 CFU per stainless steel coupon, but using the NFS instead of the cotton swab (Table 2). The recovery efficiency was determined to be 29.9%. While this method proved to be inferior to NFS protocols A and B, it performed better than the unmodified NASA standard procedure (see above).
Linearity.
The concentration of B. atrophaeus spores spotted on stainless steel coupons was decreased from nominally 100 to 3 CFU in a five-test series (100, 15, 10, 5, and 3 CFU). The NFS protocols A and B, as well as the NASA standard protocol (cotton swab), were used to sample appropriate replicates of each spore concentration. A summary of the results is provided in Table 2. Linearity in the recovery efficiencies at different CFU concentrations was demonstrated for all three methods. The weighted average for NFS protocol A was 45.4% (recovery efficiency) with a slight difference from protocol B (49.0%). In contrast, the efficiencies of the NASA standard protocol had a mean of only 13.2% recovery. No test series for the NASA standard method was accomplished with 3 CFU per coupon, as this concentration was below its limit of detection (LOD); the limit was calculated to be 7.6 spores per stainless steel coupon, based on the average recovery efficiency. For NFS protocols, the LOD was determined to be lower than 3 CFU per coupon, corresponding to 1,200 spores per m2. Using the recovery efficiency values of 45.4% for protocol A and 49.0% for protocol B, the calculated limits of detection per coupon were 2.2 and 2.0 CFU, respectively.
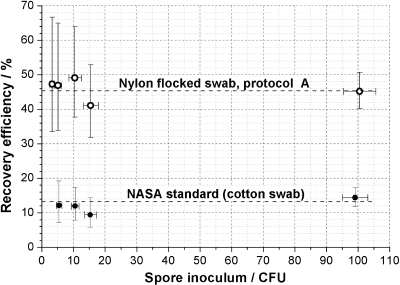
In general, the recovery efficiencies of both NFS protocols were significantly different from those of the NASA standard protocol, based on a 95% confidence interval, independent of the concentration of CFU sampled. For comparison, Fig. 1 shows the linearity of NFS protocol A and the NASA standard procedure.
FIG. 1.
Recovery efficiencies of nylon-flocked-swab protocol A and the NASA standard assay for B. atrophaeus spores at different concentrations from stainless steel coupons. The means of the recovery efficiencies are symbolized by dots. The horizontal error bars represent the confidence interval (95%) of the inoculum, and the vertical bars reflect the confidence interval of the spore recovery efficiency. The horizontal lines reflect the average recovery efficiency for each method.
Specificity.
Spores of seven different Bacillus species (approximately 100 CFU) were recovered from stainless steel coupons (Table 3). Ten replicates were performed for each species and sampling methodology (NFS protocols A and B and the NASA standard protocol). Table 3 summarizes the results and statistical analysis of the test series grouped by method, whereas Fig. 2 demonstrates the specificity of each method as histograms (grouped by species). NFS protocol A was the most efficient method for detecting Bacillus spores, with an average recovery of 35.0%. In comparison, protocol B recovered 33.4% and performed better than the NASA standard procedure, which recovered only 11.4% of the inoculated CFU. Generally, the NFS protocols were superior to the cotton swab protocol in recovering any kind of Bacillus spores. By all methods, spores of B. atrophaeus were detected with higher recovery efficiency than those of other species. No further correlations between other Bacillus species and their recovery efficiencies by different methods could be determined. Considering the extraction solution, protocol A using PBST as a storage solution had mostly higher recovery efficiencies, whereas protocol B and the NASA standard procedure (both based on water, sonication, and vortexing for extraction) detected the B. megaterium type strain and B. thuringiensis with lower efficiency. For these species, PBST without sonication and with vortexing seemed superior in removing the spores recovered from the swab material of NFS. In contrast, spores of B. megaterium 2c1 were more easily removed from the nylon material using water.
TABLE 3.
Specificity of the nylon-flocked-swab protocol compared to NASA standard protocola
| Recovery method | Actual spore inoculum (CFU; nominal, 100) | Mean recovery (CFU) | Range of recovery (CFU) | Recovery efficiency (%)b | 95% CI | Avg recovery (%)c |
|---|---|---|---|---|---|---|
| Nylon-flocked-swab protocol Ad | ||||||
| B. anthracis Sterne | 100.8 | 19.9 | 10.0-36.1 | 19.7 | 16.6-23.3 | 35.0 ± 3.2 (8.52) |
| B. atrophaeus | 100.5 | 45.4 | 35.0-55.0 | 45.2 | 40.2-50.7 | |
| B. megaterium | 88.8 | 26.3 | 15.0-55.1 | 29.6 | 25.5-34.1 | |
| B. megaterium 2c1 | 105.3 | 33.8 | 25.0-43.7 | 32.1 | 27.9-36.7 | |
| B. safensis | 103.7 | 26.8 | 32.5-48.8 | 25.8 | 22.2-29.9 | |
| B. thuringiensis | 96.1 | 36.6 | 10.0-47.6 | 38.1 | 33.9-42.7 | |
| B. thuringiensis E24 | 101.9 | 41.4 | 17.5-33.8 | 40.6 | 35.8-46.0 | |
| Nylon-flocked-swab protocol Bd | ||||||
| B. anthracis Sterne | 100.8 | 19.8 | 8.8-30.2 | 19.6 | 16.5-23.2 | 33.4 ± 5.9 (15.6) |
| B. atrophaeus | 99.1 | 50.6 | 36.2-61.2 | 51.1 | 45.9-56.8 | |
| B. megaterium | 101.0 | 20.6 | 13.7-30.0 | 20.4 | 17.3-24.0 | |
| B. megaterium 2c1 | 107.1 | 45.3 | 32.5-62.6 | 42.3 | 37.5-47.5 | |
| B. safensis | 102.5 | 26.4 | 11.3-43.8 | 25.7 | 22.1-29.9 | |
| B. thuringiensis | 89.4 | 14.8 | 7.5-22.6 | 16.5 | 13.5-20.0 | |
| B. thuringiensis E24 | 96.1 | 13.8 | 6.3-20.1 | 14.3 | 11.7-17.4 | |
| Cotton swab, NASA standard | ||||||
| B. anthracis Sterne | 100.8 | 6.5 | 2.5-12.5 | 6.5 | 4.8-8.5 | 11.4 ± 1.8 (4.66) |
| B. atrophaeus | 99.1 | 14.3 | 11.3-17.6 | 14.4 | 11.8-17.4 | |
| B. megaterium | 101.0 | 1.5 | 0.0-3.8 | 2.9 | 1.8-4.3 | |
| B. megaterium 2c1 | 107.1 | 14.6 | 6.2-21.2 | 13.7 | 11.2-16.5 | |
| B. safensis | 102.5 | 13.4 | 1.3-23.8 | 13.1 | 10.6-15.9 | |
| B. thuringiensis | 89.4 | 1.8 | 0.0-3.9 | 2.0 | 1.1-3.3 | |
| B. thuringiensis E24 | 96.1 | 3.0 | 1.3-6.3 | 3.1 | 2.0-4.7 |
Surface, stainless steel coupons; no. of replicates for each test, 10.
Recovery efficiency calculated from the mean number of recovered spores.
±Standard error of the mean (standard deviation). For further details, see Materials and Methods.
Protocol A was based on PBST for storage and vortexing for extraction; protocol B was based on water for storage and vortexing and sonification for extraction.
FIG. 2.
Recovery efficiencies for different Bacillus spores from stainless steel coupons shown in a histogram. The error bars represent the confidence intervals (95% level).
Sample surface material.
Five different sampling surfaces were compared for B. atrophaeus spore removal using NFS protocol A. The nominal inoculum was set to 100 CFU, and the results are presented in Table 4. In brief, carbon fiber-reinforced plastic and stainless steel were sampled with the highest recovery efficiencies of 62.0% and 45.2%, significantly different from each other. Roughening of the carbon fiber-reinforced plastic resulted in a significant limitation of the percentage of recovered spores to 35.4% (CFU), a decrease of 26.6% in efficiency. When sampling textiles (Vectran fabric types A and B), the recovery efficiencies were determined to be 5.9% and 8.8%, respectively.
TABLE 4.
Evaluation of nylon-flocked-swab protocol A by sampling different surfacesa
| Surface | Actual spore inoculum (CFU; nominal, 100) | No. of replicates | Mean recovery (CFU) | Range of recovery (CFU) | Recovery efficiency (%)b | 95% CI |
|---|---|---|---|---|---|---|
| Carbon fiber-reinforced plastic | 104.8 | 12 | 64.9 | 50.0-80.0 | 62.0 | 56.7-67.6 |
| Roughened carbon fiber-reinforced plastic | 104.8 | 12 | 37.1 | 20.0-53.8 | 35.4 | 31.6-39.5 |
| Stainless steel | 100.5 | 10 | 45.4 | 35.0-55.0 | 45.2 | 40.2-50.7 |
| Vectran fabric type A | 104.8 | 20 | 6.1 | 2.5-10.0 | 5.9 | 4.7-7.2 |
| Vectran fabric type B | 104.8 | 18 | 9.2 | 5.0-15.0 | 8.8 | 7.3-10.4 |
Spores, B. atrophaeus.
Recovery efficiency calculated from the mean number of recovered spores.
Direct inoculation.
The extraction efficiency of spores from the NFS was determined by direct inoculation of a nominal 100 (actual, 99.1) CFU onto the swab head. Extraction was carried out according to protocol B without 1 h of storage, resulting in an efficiency of 80.1%, whereas the confidence intervals (95%) ranged from 73.0% to 87.6%.
Worker variability.
Compared to an average recovery efficiency of 45.4% (protocol A), the inexperienced experimenter achieved an efficiency of 39.3% in one test series of 100 CFU when sampling stainless steel coupons (Table 2).
Microscopic analyses.
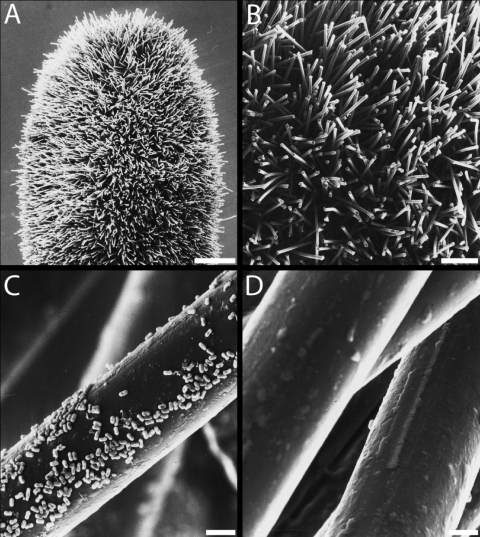
SEM techniques allowed close examination of nylon-flocked-swab (Fig. 3) and cotton swab (Fig. 4) materials prior to and after sampling and extraction. The NFS showed many single fibers protruding from the swab head (Fig. 3A and B). They were homogeneous in size, with a diameter of approximately 20 μm. Sampling did not affect the integrity of the swab or its fibers, but extraction (according to protocol A) sometimes caused agglutination of the fibers. After sampling a stainless steel coupon with 108 highly purified spores, many of them were visible on the nylon fibers, and no aggregation of spores was observed (Fig. 3C). Their successful removal was observed in SEM images captured after extraction (Fig. 3D), which correlated with results gained from direct inoculation (see above).
FIG. 3.
Scanning electron micrograph of the nylon-flocked swab. (A) Swab head prior to sampling. Bar, 1 mm. (B) Enlarged view of panel A. Bar, 200 μm. (C) Fiber from the swab after sampling stainless steel coupons showing many B. atrophaeus spores. Bar, 5 μm. (D) Fibers of the swab after extraction. Only a few isolated spores appeared. Bar, 5 μm.
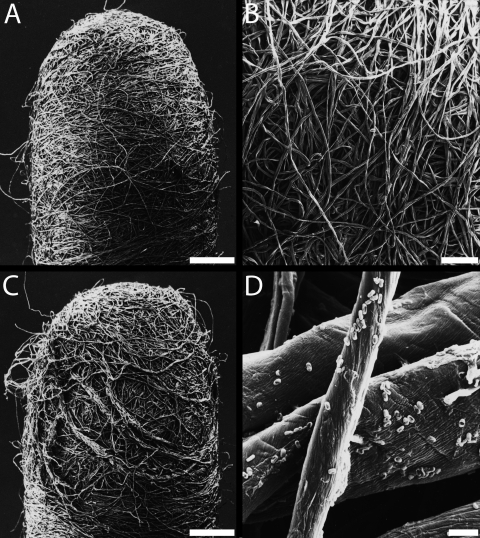
FIG. 4.
Scanning electron micrograph of the cotton swab. (A) Swab head prior to sampling. Bar, 1 mm. (B) Enlarged view of panel A. Bar, 200 μm. (C) Disintegrated swab head after sampling. Bar, 1 mm. (D) Enlarged view of panel C showing some B. atrophaeus spores on the cotton fibers. Bar, 5 μm.
The SEM magnification of cotton swabs revealed that the head consisted of many unordered cotton fibers wrapped around the swab head (Fig. 4A and B). Due to their organic nature, the thicknesses of the fibers varied from about 5 to 50 μm. After sampling stainless steel coupons (108 spores), the integrity of the swab was lost, resulting in many fibers protruding from the fiber network (Fig. 4C). After extraction, the swab head doubled in size due to high absorption of water. Occasionally, this preparation revealed spores on the fiber network even after extraction.
DISCUSSION
This study evaluated the efficiency of an alternative swab method for assessing spore contamination on different spacecraft-associated surfaces. The current NASA standard method for the recovery of spores from spacecraft surfaces is based on a sampling procedure using cotton swabs, spore extraction via sonication and vortexing, and enumeration by TSA pour plating of the (spore) suspension obtained (26). For future ESA (Mars) missions, a modification of the protocol due to different mission requirements and conditions will be necessary. The NFS was chosen to replace the problematic cotton swab, based on preliminary comparative tests of different swabs and the experiments of Dalmaso et al. (12), which demonstrated more efficient detection of vegetative contaminants on pharmaceutically relevant surfaces. Further modifications of the NASA standard procedure included reduction of the extraction volume, the cultivation procedure, and the addition of an alternate protocol adapted to the recovery of vegetative cells (protocol A) (Table 1).
The overall recovery efficiency of B. atrophaeus spores (CFU) from stainless steel coupons using NFS protocols A and B (45.4% and 49.0%) revealed a 3- to 4-fold increase compared to the NASA standard procedure (13.2%). The results for protocols A and B were quite similar, indicating that neither the selection of storage solution nor the use of sonication significantly affected B. atrophaeus spore recovery. Tests combining the NASA standard method with the NFS detected 29.9% of B. atrophaeus spores (CFU), establishing that not only the NFS itself, but also the chosen laboratory method, increased the detection of spores.
Other methods of B. atrophaeus spore recovery from stainless steel have been reported in recent literature; the BiSKit (QuickSilver Analytics, Abingdon, MD), for example, has been utilized to sample areas of 1 m2 with an efficiency of about 47.3% (9). Although it was used in the past to sample clean-room environments (23, 28), it could not be adapted to spacecraft sampling due to electrical-discharge issues and residue problems (28). A low recovery efficiency of 28.2% was reported by Brown et al. (5) for a vacuum filter sock sampler, and it was therefore not considered for spacecraft cleanliness control. Another method for large-area sampling using a polyester-rayon wipe performed with 34.6% efficiency, having lower recovery efficiencies than the nylon-flocked-swab protocols (4). Furthermore, the average recovery efficiencies of the nylon-flocked-swab protocols were higher than those of a reported rayon swab protocol at 41.4% (6).
Inoculating nylon-flocked swabs directly with approximately 100 CFU resulted in 80.1% recovery efficiency, which is lower than those described for other swabs using B. anthracis Sterne as a model organism (ranging from 81.9 to 96.6% [32]). Other studies evaluating rayon swabs and polyester-rayon blends reported extraction efficiencies of 75.6% and 93.2%, respectively, for B. atrophaeus spores (4, 6). Scanning electron micrographs of the nylon-flocked and the cotton swabs revealed that a small number of spores were left on the swab surfaces after sampling and extraction. For cotton swabs, release of 93.1% of B. anthracis spores has been reported (32). To avoid leftover spores on the swab material and to increase spore detection, some previous studies were done with alginate swabs, which dissolve completely in sodium hexametaphosphate. However, due to low efficiency in spore recovery and growth inhibition of, e.g., B. globigii (currently B. atrophaeus), these swabs were considered to be less applicable to spacecraft bioburden control (2, 39).
In this study, the maximum recovery efficiency of B. atrophaeus spores from surfaces was detected on carbon fiber-reinforced plastic (62.0%). In combination with an extraction efficiency of 80.1%, the data indicated that only a low number of spores remained on the swabbed surface (∼18%). Previous publications have suggested that spores may become fixed when applied in alcohol solution due to evaporation of the solvent (32). More efficient removal from the surface may be achieved with a different spore application method, as already described for powder preparations, but it has the disadvantage of nonhomogeneous spore distribution (4-6).
In terms of handling, the nylon-flocked-swab protocols were superior to the NASA standard swab. This was mainly because the NFS was provided with a predetermined breaking point, ensuring easy removal of the swab head for extraction after sampling. In contrast, the cotton swab had a wooden shaft without a breaking point, requiring the use of a (sterile) cutter to avoid imprecise breaking. Additionally, the disintegration of the cotton swab throughout sampling emphasizes the potential for fiber residue problems when sampling spacecraft components.
In general, the poor precision in swab experiments has been suggested to be not only an inherent characteristic of sampling, but one that is exacerbated by operator collection technique, such as the angle and pressure applied to the surface. Other cited errors contributing to poor precision include inconsistent spore release from swabs due to variations in vortexing, sonication, or pipetting, and colony counting errors (2, 32). It has been suggested that variations of experiments in laboratory settings are minimal compared to those in application due to a variety of different factors (2). However, this study was performed with only two scientists as experimenters, and only one performed all specificity and linearity studies. Parallel data for the 100-CFU recovery from stainless steel by an inexperienced person revealed a recovery efficiency for protocol A of 39.3%, while the experienced scientist achieved an efficiency of 45.2%. These data reiterate that prior experience in handling and swabbing could increase recovery efficiency, but they also emphasize the robustness of our method, since the decreased efficiency of the “untrained” test person was marginal.
The general guideline for the evaluation of NFS protocols A and B was USP 1223 (3), and stainless steel coupons served as the reference surface. Accuracy, precision, and linearity have been shown for different spore concentrations with an appropriate number of experiments. The recovery efficiency varied only slightly within the tested range. Finally, to determine the specificity of the method, the validation of the nylon-flocked-swab protocol was performed for a broad variety of seven Bacillus species. The protocols could be used for all of the species tested, and the NFS protocols recovered more spores than the NASA standard procedure, regardless of the spore species examined. As spores from different species resulted in diverging recovery efficiencies between NFS protocols A and B, further investigation is necessary to clarify whether the use of PBST or water as the extraction solution results in greater recoveries. In all methods for all species tested, B. atrophaeus was removed from stainless steel coupons with the highest efficiency. Nevertheless, the reason for variations in recovery efficiencies is unclear. It is possible that different physiochemical adhesive properties, like hydrophobicity or the molecular composition of spore sheaths, affect the release of spores from surfaces (31). For instance, B. anthracis possesses an exosporium, while B. atrophaeus does not (17).
With regard to B. anthracis Sterne spore recovery from stainless steel, NFS protocols A and B exhibited identical recovery rates (∼20%) and outperformed the present NASA standard protocol (6.5%). Compared to other efficiencies reported in the literature, the values for the NFS protocols correlated with those described by Estill et al. (14), which ranged from 3.7 to 18.0% recovery efficiency, depending on the method applied. Nevertheless, other studies demonstrated higher efficiency of B. anthracis spore recovery from stainless steel (up to 49.1%) when other swab materials were used (18, 32). Interestingly, the efficiency for cotton swabs was reported to be even higher (41.7%) when different methods for extraction were applied (32). One conclusion that can be drawn is that the present NASA protocol is deficient in extracting B. anthracis spores from swab material. In past studies, B. atrophaeus was used as a surrogate to determine an appropriate spore recovery method for B. anthracis (4-6, 8, 9, 13). In this study, three different protocols, using two different swabs, consistently detected B. atrophaeus with a 2-fold increase on stainless steel surfaces. Hence, the results in this communication suggest that recovery efficiencies using B. atrophaeus spores as a surrogate should be regarded with skepticism concerning their transferability to B. anthracis contamination.
Previous investigations describing spore removal from different surfaces have lacked consensus. Two studies evaluating a vacuum filter sock sampler and rayon swabs reported no significant difference in spore detection when the surfaces were varied (5, 6). Conversely, Buttner et al. (8) reported an alteration of efficiency when the wipe-rinse technique was applied to porous and nonporous surfaces. The results of this study affirmed these observations, since the recovery efficiency of the NFS protocol varied from 5.9% to 62.0%, depending on the surfaces sampled: the yield of spore detection was correlated with the porosity of the surface. For instance, carbon fiber-reinforced plastic was the smoothest surface, resulting in the highest recovery efficiencies (62.0%), but it showed a loss of detection of 26.6% when its surface was roughened. Vectran fabric types A and B proved to be even more problematic for recovery of spores, with only 5.9% and 8.8% recovery efficiencies. Differences in surface textural and physiochemical adhesive properties may be the reason for significant variations in the recovery efficiencies of the different surfaces (35) and, in particular, of Vectran fabric types. On the other hand, a recent publication demonstrated that different seeding methods for spores (dry or liquid deposition) can significantly affect sampling success (13). In the past, Vectran textiles have been used for different NASA Mars landing missions: Pathfinder (http://marsrover.nasa.gov/mission/spacecraft_edl_airbags.html) and Mars Exploration Rover (http://marsprogram.jpl.nasa.gov/MPF/mpf/mpfairbags.html). The bioburden control of these missions was carried out according to NASA standard protocols. Historically, knowledge about the potential threat concerning forward contamination of Mars arising from undetected spores on these surfaces has been very limited. Further investigation is necessary and warranted to clarify whether there is a proper sampling method for bioburden determination on these “problematic” fabrics.
A recent study showed the successful application of the NFS in the field of planetary protection for identification of facultatively and strictly anaerobic microorganisms (38). Also, Dalmaso and coworkers demonstrated broad application of the swab in sampling stainless steel coupons inoculated with different vegetative microorganisms, such as Bacillus, Candida, and Aspergillus (12). Recovery efficiencies ranged from 42 to 66%, depending on the species inoculated. By comparing these results to those gained in this study, the recovery of Bacillus spores was lower—but this is a qualified statement due to the differences in inoculation and in processing of the samples. In the past, many bacilli were determined to be associated with spacecraft assembly facilities (21, 22, 25, 40). Nevertheless, the applicability of the NFS for other clean-room contaminants, like spore-forming Paenibacillus or the anaerobic Clostridium, has not been demonstrated yet. Furthermore, its practicality for molecular microbial community analysis is still outstanding and required. Nevertheless, present planetary-protection requirements are based on the determination of aerobic bacterial spore load but do not include the detection of mesophilic, vegetative cells or anaerobic sporeformers or molecular analysis (11).
The results of this study showed the superiority of two NFS protocols over the present NASA standard procedure for detection of different Bacillus spores. From a public health perspective, the recovery efficiencies for B. anthracis spores may lead to more accurate detection of their presence in the event of a B. anthracis scare. However, although its application to surfaces with different roughness resulted in high recovery efficiencies for B. atrophaeus spores, the data also showed insufficient recovery of spores from rough fabrics. Finally, the introduction of the nylon-flocked-swab protocols to the field of planetary protection will significantly contribute to better cleanliness control and help to preserve the scientific integrity of present and future life detection missions.
Acknowledgments
We are grateful to ESA for funding this project (contract no. 21942/08/NL/MP).
Gerhard Kminek (ESA planetary protection officer), Marco Wolf, and Joerg Bolz are acknowledged for critical inputs and discussion. We gratefully thank Petra Rettberg for providing Bacillus strains, EADS Astrium (Bremen, Germany) for providing surface sample material, and Angelika Kuehn for support and maintenance of the scanning electron microscope. Karoline Wuensche is acknowledged for contributing to this study and Shariff Osman and Lauren Valentor for critical proofreading.
Footnotes
Published ahead of print on 11 June 2010.
REFERENCES
- 1.Abu-Diab, A., M. Azzeh, R. Ghneim, M. Zoughbi, S. Turkuman, N. Rishmawi, A. E. Issa, I. Siriani, R. Dauodi, R. Kattan, and M. Y. Hindiyeh. 2008. Comparison between pernasal flocked swabs and nasopharyngeal aspirates for detection of common respiratory viruses in samples from children. J. Clin. Microbiol. 46:2414-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelotti, R., M. J. Foter, K. A. Busch, and K. H. Lewis. 1958. A comparative evaluation of methods for determining the bacterial contamination on surfaces. Food Res. 23:175-185. [Google Scholar]
- 3.Anonymous. 2003. USP 1223: validation of alternative microbiological methods. Pharm. Forum 29:256-264. [Google Scholar]
- 4.Brown, G. S., R. G. Betty, J. E. Brockmann, D. A. Lucero, C. A. Souza, K. S. Walsh, R. M. Boucher, M. Tezak, M. C. Wilson, and T. Rudolph. 2007. Evaluation of a wipe surface sample method for collection of Bacillus spores from nonporous surfaces. Appl. Environ. Microbiol. 73:706-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, G. S., R. G. Betty, J. E. Brockmann, D. A. Lucero, C. A. Souza, K. S. Walsh, R. M. Boucher, M. S. Tezak, and M. C. Wilson. 2007. Evaluation of vacuum filter sock surface sample collection method for Bacillus spores from porous and non-porous surfaces. J. Environ. Monit. 9:666-671. [DOI] [PubMed] [Google Scholar]
- 6.Brown, G. S., R. G. Betty, J. E. Brockmann, D. A. Lucero, C. A. Souza, K. S. Walsh, R. M. Boucher, M. S. Tezak, M. C. Wilson, T. Rudolph, H. D. Lindquist, and K. F. Martinez. 2007. Evaluation of rayon swab surface sample collection method for Bacillus spores from nonporous surfaces. J. Appl. Microbiol. 103:1074-1080. [DOI] [PubMed] [Google Scholar]
- 7.Bruckner, J., and K. Venkateswaran. 2007. Overview of methodologies to sample and assess microbial burden in low biomass environment. Jpn. J. Food Microbiol. 24:61-70. [Google Scholar]
- 8.Buttner, M. P., P. Cruz-Perez, and L. D. Stetzenbach. 2001. Enhanced detection of surface-associated bacteria in indoor environments by quantitative PCR. Appl. Environ. Microbiol. 67:2564-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buttner, M. P., P. Cruz, L. D. Stetzenbach, A. K. Klima-Comba, V. L. Stevens, and P. A. Emanuel. 2004. Evaluation of the Biological Sampling Kit (BiSKit) for large-area surface sampling. Appl. Environ. Microbiol. 70:7040-7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chernesky, M., S. Castriciano, D. Jang, and M. Smieja. 2006. Use of flocked swabs and a universal transport medium to enhance molecular detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J. Clin. Microbiol. 44:1084-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Commitee on Space Research. 2005. COSPAR planetary protection policy. COSPAR/IAU Workshop on Planetary Protection, October 2002 ed., amended 2005. Commitee on Space Research (COSPAR), International Council for Science, Paris, France. http://cosparhq.cnes.fr/Scistr/Pppolicy.htm.
- 12.Dalmaso, G., M. Bini, R. Paroni, and M. Ferrari. 2008. Qualifiction of high-recovery, flocked swabs as compared to traditional rayon swabs for microbiological environmental monitoring of surfaces. J. Pharm. Sci. Technol. 63:191-199. [PubMed] [Google Scholar]
- 13.Edmonds, J. M., P. J. Collett, E. R. Valdes, E. W. Skowronski, G. J. Pellar, and P. A. Emanuel. 2009. Surface sampling of spores in dry-deposition aerosols. Appl. Environ. Microbiol. 75:39-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Estill, C. F., P. A. Baron, J. K. Beard, M. J. Hein, L. D. Larsen, L. Rose, F. W. Schaefer III, J. Noble-Wang, L. Hodges, H. D. Lindquist, G. J. Deye, and M. J. Arduino. 2009. Recovery efficiency and limit of detection of aerosolized Bacillus anthracis Sterne from environmental surface samples. Appl. Environ. Microbiol. 75:4297-4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Cooperation for Space Standardization. 2008. Microbial examination of flight hardware and cleanrooms. ECSS-Q.-ST-70-55C. European Cooperation for Space Standardization (ESA-ESTEC), Noordwijk, Netherlands.
- 16.Fast, P. G. 1972. The σ-endotoxin of Bacillus thuringiensis III: a rapid method for separating parasporal bodies from spores. J. Invertebr. Pathol. 20:139-140. [Google Scholar]
- 17.Hachisuka, Y., K. Kojima, and T. Sato. 1966. Fine filaments on the outside of the exosporium of Bacillus anthracis spores. J. Bacteriol. 91:2382-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodges, L. R., L. J. Rose, A. Peterson, J. Noble-Wang, and M. J. Arduino. 2006. Evaluation of a macrofoam swab protocol for the recovery of Bacillus anthracis spores from a steel surface. Appl. Environ. Microbiol. 72:4429-4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirschner, L. E., and J. R. Puleo. 1979. Wipe-rinse technique for quantitating microbial contamination on large surfaces. Appl. Environ. Microbiol. 38:466-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krech, T., S. Castriciano, D. Jang, M. Smieja, G. Enders, and M. Chernesky. 2009. Detection of high risk HPV and Chlamydia trachomatis in vaginal and cervical samples collected with flocked nylon and wrapped rayon dual swabs transported in dry tubes. J. Virol. Methods 162:291-293. [DOI] [PubMed] [Google Scholar]
- 21.La Duc, M. T., A. Dekas, S. Osman, C. Moissl, D. Newcombe, and K. Venkateswaran. 2007. Isolation and characterization of bacteria capable of tolerating the extreme conditions of clean room environments. Appl. Environ. Microbiol. 73:2600-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.La Duc, M. T., R. Kern, and K. Venkateswaran. 2004. Microbial monitoring of spacecraft and associated environments. Microb. Ecol. 47:150-158. [DOI] [PubMed] [Google Scholar]
- 23.La Duc, M. T., S. Osman, P. Vaishampayan, Y. Piceno, G. Andersen, J. A. Spry, and K. Venkateswaran. 2009. Comprehensive census of bacteria in clean rooms by using DNA microarray and cloning methods. Appl. Environ. Microbiol. 75:6559-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moeller, R., G. Horneck, R. Facius, and E. Stackebrandt. 2005. Role of pigmentation in protecting Bacillus sp. endospores against environmental UV radiation. FEMS Microbiol. Ecol. 51:231-236. [DOI] [PubMed] [Google Scholar]
- 25.Moissl, C., S. Osman, M. T. La Duc, A. Dekas, E. Brodie, T. DeSantis, and K. Venkateswaran. 2007. Molecular bacterial community analysis of clean rooms where spacecraft are assembled. FEMS Microbiol. Ecol. 61:509-521. [DOI] [PubMed] [Google Scholar]
- 26.National Aeronautics and Space Administration. 1999. NASA standard procedures for the microbiological examination of space hardware, NPG 5340.1D. Jet Propulsion Laboratory Communication. National Aeronautics and Space Administration, Pasadena, CA.
- 27.Pfanzagl, J., and F. Puntigam. 1961. Aussagen über den Quotienten zweier Poisson-Parameter und deren Anwendungen auf ein Problem der Pockenschutzimpfung. Biom. Z. 3:135-142. [Google Scholar]
- 28.Probst, A., P. Vaishampayan, S. Osman, C. Moissl-Eichinger, G. L. Andersen, and K. Venkateswaran. 2010. Diversity of anaerobic microbes in spacecraft assembly clean rooms. Appl. Environ. Microbiol. 76:2837-2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Przyborowski, J., and H. Wilenski. 1940. Homogeneity of results in testing samples from Poisson series. Biometrika 31:313-323. [Google Scholar]
- 30.Puleo, J. R., N. D. Fields, S. L. Bergstrom, G. S. Oxborrow, P. D. Stabekis, and R. Koukol. 1977. Microbiological profiles of the Viking spacecraft. Appl. Environ. Microbiol. 33:379-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rönner, U., U. Husmark, and A. Henriksson. 1990. Adhesion of Bacillus spores in relation to hydrophobicity. J. Appl. Bacteriol. 69:550-556. [DOI] [PubMed] [Google Scholar]
- 32.Rose, L., B. Jensen, A. Peterson, S. N. Banerjee, and M. J. Srduino. 2004. Swab materials and Bacillus anthracis spore recovery from nonporous surfaces. Emerg. Infect. Dis. 10:1023-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sahai, H., and S. C. Misra. 1992. Comparing means of two Poisson distributions. Math. Sci. 17:60-67. [Google Scholar]
- 34.Schuerger, A. C., R. L. Mancinelli, R. G. Kern, L. J. Rothschild, and C. P. McKay. 2003. Survival of endospores of Bacillus subtilis on spacecraft surfaces under simulated Martian environments: implications for the forward contamination of Mars. Icarus 165:253-276. [DOI] [PubMed] [Google Scholar]
- 35.Schuerger, A. C., J. T. Richards, P. E. Hintze, and R. G. Kern. 2005. Surface characteristics of spacecraft components affect the aggregation of microorganisms and may lead to different survival rates of bacteria on Mars landers. Astrobiology 5:545-559. [DOI] [PubMed] [Google Scholar]
- 36.Schuerger, A. C., S. Trigwell, and C. I. Calle. 2008. Use of non-thermal atmospheric plasmas to reduce the viability of Bacillus subtilis on spacecraft surfaces. Int. J. Astrobiol. 7:47-57. [Google Scholar]
- 37.Smith, P. H., L. K. Tamppari, R. E. Arvidson, D. Bass, D. Blaney, W. V. Boynton, A. Carswell, D. C. Catling, B. C. Clark, T. Duck, E. Dejong, D. Fisher, W. Goetz, H. P. Gunnlaugsson, M. H. Hecht, V. Hipkin, J. Hoffman, S. F. Hviid, H. U. Keller, S. P. Kounaves, C. F. Lange, M. T. Lemmon, M. B. Madsen, W. J. Markiewicz, J. Marshall, C. P. McKay, M. T. Mellon, D. W. Ming, R. V. Morris, W. T. Pike, N. Renno, U. Staufer, C. Stoker, P. Taylor, J. A. Whiteway, and A. P. Zent. 2009. H2O at the Phoenix landing site. Science 325:58-61. [DOI] [PubMed] [Google Scholar]
- 38.Stieglmeier, M., R. Wirth, G. Kminek, and C. Moissl-Eichinger. 2009. Cultivation of anaerobic and facultatively anaerobic bacteria from spacecraft-associated clean rooms. Appl. Environ. Microbiol. 75:3484-3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strong, D. H., M. J. Woodburn, and M. M. Mancini. 1961. Preliminary observations on the effect of sodium alginate on selected nonsporing organisms. Appl. Microbiol. 9:213-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Venkateswaran, K., M. Satomi, S. Chung, R. Kern, R. Koukol, C. Basic, and D. White. 2001. Molecular microbial diversity of a spacecraft assembly facility. Syst. Appl. Microbiol. 24:311-320. [DOI] [PubMed] [Google Scholar]