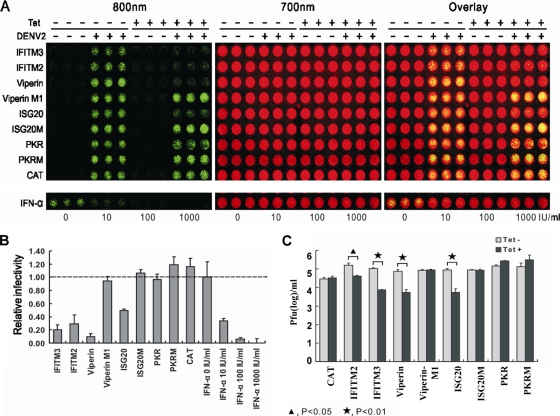
FIG. 4.
Effects of ISGs on DENV infection. (A) The stable cell lines that express CAT protein or ISGs and the respective mutant proteins were seeded into the wells of a 96-well plate at a density of 5 × 104 per well. At 24 h postseeding, the cells were cultured in the absence or presence of 1 μg/ml tetracycline for 24 h, infected with serotype 2 DENV at a multiplicity of infection of 0.1, and refed with medium with or without tetracycline. As controls, parental FLP-IN T Rex cells were left untreated or pretreated with the indicated concentrations of IFN-α for 24 h, infected with DENV-2, and cultured further in medium with or without the indicated concentrations of IFN-α. At 48 h after infection, cells were fixed with 3.7% formaldehyde in phosphate-buffered saline, incubated with anti-E antibody 4G2 (1:200; Millipore) for 1 h, and incubated with an anti-mouse IRDye 800CW-labeled secondary antibody together with two reagents for cell staining (DRAQ5 from Biostatus and Sapphire700 from LI-COR). The DENV E protein was visualized in LI-COR Odyssey in the 800-nm channel as green. The cell viability was determined by Sapphire 700 staining (Red color). (B) E protein levels were determined with LI-COR Odyssey and normalized to cell viability. The relative infectivity represents the mean ± the standard deviation (n = 3) of the ratio of the density obtained from wells treated with IFN-α or cultured in the presence of tetracycline to that obtained from wells that were left untreated or cultured in the absence of tetracycline. (C) Effects of ISGs on DENV production. The indicated cell lines were cultured in the absence or presence of 1 μg/ml tetracycline (Tet) for 24 h, infected with DENV-2 at a multiplicity of infection of 0.1, refed with medium with or without tetracycline, and cultured for 3 days. The culture medium was changed every 24 h, and virus yields between 48 and 72 h postinfection were determined by plaque assay and expressed as PFU per milliliter of culture medium (n = 3).