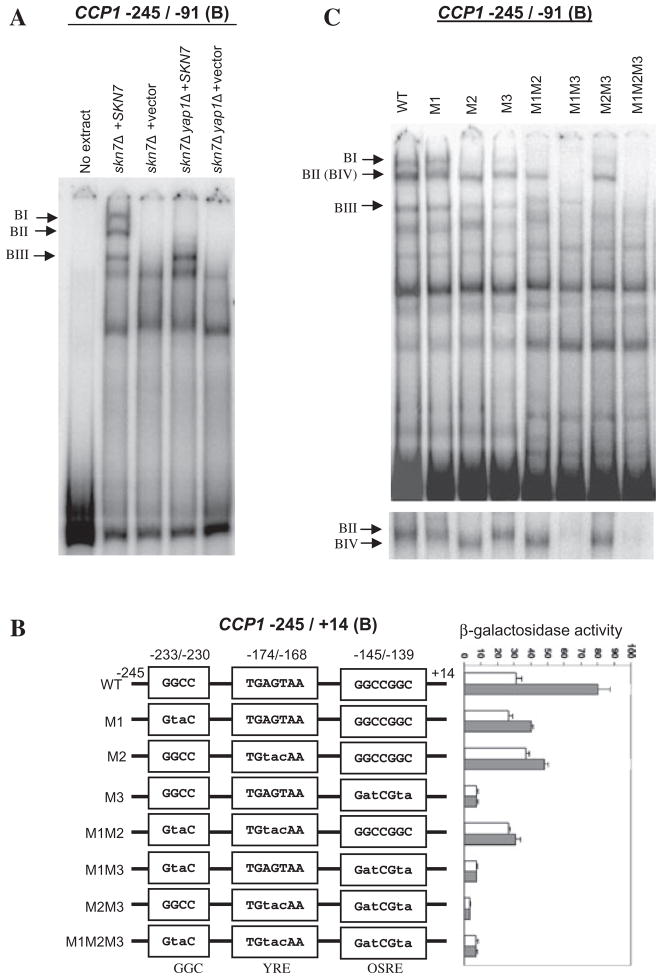
Fig. 2.
Identification of three elements between −245 and −91 (fragment B) of CCP1 that contribute to the oxidative stress response.
A. Electrophoretic mobility shift assays showing the formation of Skn7p- and Yap1p-dependent complexes with CCP1 promoter fragment B. Extracts prepared from skn7Δ (JF1904) and skn7Δyap1Δ (JF2312) strains carrying the SKN7 plasmid pSL232 (Li et al., 1998) or empty vector, pRS315, were mixed with labelled probe corresponding to −245 to −91 (fragment B) of the CCP1 promoter. Complexes were separated by non-denaturing polyacrylamide gel electrophoresis at 200 V for 6 h. Skn7p- and Yap1p-dependent complexes are labelled to the left.
B. The sequence of the GGC-containing sequence (−233/−230), the potential YRE (−174/−168) and OSRE (−145/−139) are shown in three separate boxes in the line drawing. The sequence changes in mutants M1, M2 and M3 are indicated by lower case letters. Each fragment was fused to the lacZ gene in pSEYC102 (gift from S. Moye-Rowley, University of Iowa) and the activity of each reporter construct was assayed in the skn7Δ strain (JF1904) carrying the SKN7 expression plasmid, pSL232 (Li et al., 1998). Strains were untreated (white bars) or treated with 0.3 mM H2O2 (shaded bars). β-Galactosidase activity is given in Miller units (Miller, 1972) and is the average of three or four independent transformants. Error bars represent the standard deviation of the mean.
C. Electrophoretic mobility shift assays using fragments containing wild-type or mutated (M1, M2, M3) CCP1 promoter sequences. Extracts were from the skn7Δ strain (JF1904) carrying the SKN7 expression plasmid, pSL232 (Li et al., 1998). Skn7p- and Yap1p-dependent complexes are labelled to the left. The image shown at the bottom was from a gel run for 6 h to resolve complexes II and IV.