Abstract

Synthesis and properties of a new family of π-extended dipyrrins, capable of forming brightly fluorescent complexes with metal ions, are reported. The metal complexes posses tunable spectral bands and exhibit different emission properties depending on the mode of metal coordination.
Boron dipyrrins (BODIPY) form a popular group of fluorophores due to their high emission quantum yields, excellent photostability and versatile chemistry.1 While boron complexes of dipyrrins are by far the most recognized, dipyrrins have also long been known to form stable adducts with metal ions.2,3,4 Metallodipyrrins are typically isolated as homoleptic bis-complexes (ML2, L=dipyrrin), which mainly find use as building blocks for construction of various supramolecular assemblies.5 Curiously, unlike BODIPY, homoleptic metallodipyrrins practically do not fluoresce, although there are several important exemptions.6 For example, Holten, Lindsey et al showed that by increasing the size of the meso-aryl group in Zn bis-aryldipyrrins, the non-radiative decay could be diminished, affording considerable gain in emission.6a On the other hand, some recently reported heteroleptic Al3+ and Sn2+ mono-dipyrrinates (MLXn) exhibit bright fluorescence,7,8 suggesting that the emissivity of metallodipyrrins could in part be related to the mode of metal coordination. Overall, the interplay between the structure and photophysics of metallodipyrrins is not well understood; and generally metallodipyrrins are considered poorly emissive species.1a
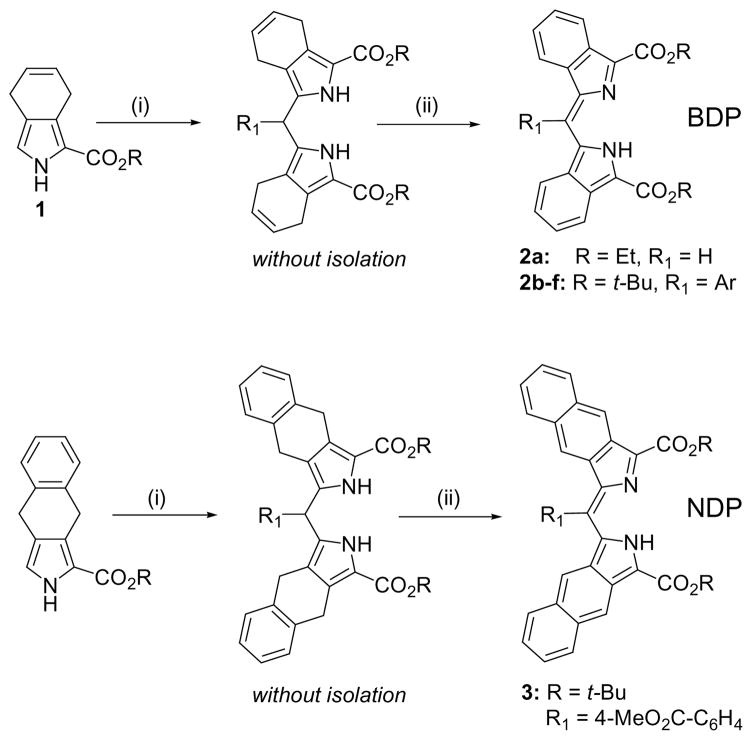
Here we report a new family of aromatically π-extended dipyrrin molecules capable of forming bright fluorescent complexes with metal ions and exhibiting a unique fluorescence modulation effect mediated by exciton coupling.9 A recently described approach to π-extended porphyrins, based on 4,7-dihydroisoindole and its derivatives (Scheme 1),10 paved a new way to π-extended oligopyrroles, including 2,2′-alkoxycarbonyl-dibenzo- (BDP) and dinaphtho[2,3]dipyrrins (NDP). A simple one-pot procedure leading to BDP’s relies on the condensation of 2-substituted 4,7-dihydroisoindole 1 with aldehydes,10c followed by the oxidation of dipyrromethanes with DDQ. Similarly, dinaphthodipyrrins can be synthesized from the corresponding pyrrole-esters reported previously (Scheme 1).10a
Scheme 1.
Reagents and conditions: (i) R1CHO, p-toluenesulfonic acid, Bu4NCl, CH2Cl2, r.t., 12–24 h; (ii) DDQ (3 eq.), THF, r.t., 10–30 min. Yields: 2a, 93%; 2b (R1 = 3,5-t-Bu2C6H3), 88%; 2c (R1 = 4-BrC6H4), 92%; 2d (R1 = 4-MeO2CC6H4), 87%; 2e (R1 = 3,5-(MeO)2C6H3), 84%; 2f (R1 = 2-thienyl), 82%; 3, 78%.
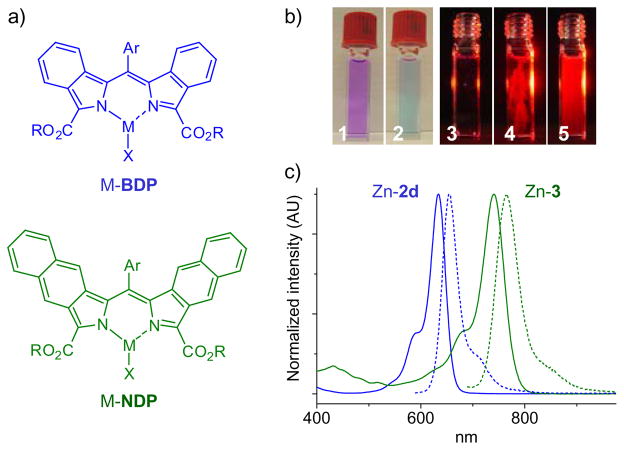
All the synthesized dipyrrins exhibit broad absorption bands (ε~4–7×104 M−1cm−1) ranging from 550–570 nm for BDP’s (2a–f) to 660–700 nm for NDP (3). Taken as free-bases, BDP’s and NDP’s fluoresce very weakly (φfl~0.01–0.02 at 22°C); however, upon addition of metal salts (e.g. Zn(OAc)2, Ca(OAc)2, YCl3, LaCl3, GdCl3) their solutions instantly become fluorescent. For example, upon addition of Zn(OAc)2, purple solutions of BDP’s in DMF immediately change color to deep blue with appearance of bright red fluorescence (Fig. 1b). In contrast, metallation of regular dipyrrins typically requires heating and/or presence of bases and leads to non-emissive products.
Figure 1.
(a) Structures of heteroleptic metal complexes of BDP’s and NDP’s. (b) Color changes upon addition of Zn(OAc)2 to a solution of 2a in DMF (1,2). The solution was illuminated with red LED (λmax=635 nm) from the side (3), a few crystals of Zn(OAc)2 were added (4), the solution was shaken (5). (c) Absorption and emission spectra of Zn complexes of BDP 2d (blue) and NDP 3 (green). Complexes were prepared in situ by adding Zn(OAc)2 to solutions of free-base dipyrrins (~5×10−6 M) in DMF (X=OAc).
The optical transitions of the new π-extended dipyrrinates span the entire red spectral range (Fig. 1a), resembling in shape the bands of BODIPY, dibenzo-BODIPY12 and dibenzo-aza-BODIPY.13 The emission quantum yields reach as high as 0.7 in the case of ZnBDP’s (Table 1). The fluorescence quantum yields of NDP complexes are somewhat lower, reflecting a ~100 nm red-shift in the NDP transition and subsequent enhancement of the non-radiative decay.14 The high emissivity of BDP and NDP complexes is probably related to the structures of π-extended dipyrrin ligands themselves, which are different that other dipyrrins reported to date. By comparison, in similarly π-extended porphyrins, the S1→S0 radiative rate was found to be higher than in the corresponding non-aromatically extended analogues.15 From the practical point of view, red absorption bands of BDP’s and NDP’s and their strongly fluorogenic complexation make these dipyrrins promising as probes for biological sensing of metal ions (e.g. Zn2+).
Table 1.
Optical properties of Zn and Ca complexes of π-extended dipyrrins in DMF.a
| Comp. | Abs λmax, nm | Emiss λmax, nm | φflb | Comp. | Abs λmax, nm | Emiss λmax, nm | φflb |
|---|---|---|---|---|---|---|---|
| Zn-2a | 637 | 650 | 0.70 | Ca-2a | 639 | 650 | 0.64 |
| Zn-2d | 631 | 639 | 0.65 | Ca-2d | 638 | 645 | 0.58 |
| Zn-3 | 740 | 761 | 0.08 | Ca-3 | 737 | 762 | 0.05 |
Complexes were generated in situ upon addition of metal acetates (ca 10-fold excess) to solutions of dipyrrins;
relative to Rhodamine 6G in EtOH (φfl=0.94).11
A remarkable feature of the observed complexation is complete on-off switching of fluorescence depending on the mode of metal coordination. For example, when BDP 2d reacted with Zn(OAc)2 in acetone, an intensely fluorescent solution formed instantly (Fig. 1b); but after a few hours the fluorescence disappeared and dark blue crystals precipitated. These crystals were poorly soluble in acetone, but well soluble in toluene, CH2Cl2 or pyridine. The solutions revealed different absorption spectra (Fig. 2a) and practically no fluorescence. The crystals were unambiguously identified as the homoleptic ML2 complex (S21). Upon reaction of this complex with excess of Zn salt (in pyridine), the spectrum again adopted the BODIPY-like shape, and the fluorescence was fully regained (Fig. 2a). The conversion could be followed by NMR (Fig. 2b, S17), absorption and fluorescence (S17).
Figure 2.

(a) Changes in the absorption and emission spectra (λex=560 nm) of Zn-2d (ML2) upon addition of Zn(OAc)2 (excess). ML2 in pyridine before addition of Zn(OAc)2 (black), 2 h (red) and 16 h (blue) after addition. Inset: exciton splitting effect; line thickness reflects the transition probability. (b) Aromatic regions of the 1H NMR spectra of ML2 and MLX forms of Zn-2d in pyridine-d5 (solvent resonances are marked in blue, assignments are based on 1H COSY experiments (S7) and refer to the structure on the right). (c) Optimized structure of homoleptic Zn-2e complex (B3LYP/6-31G(d), pendant groups are omitted for clarity). Dotted arrows show orientation of the transition dipole moments (μ1 and μ2) of the individual dipyrrin units.
The observed spectral changes most likely originate from the solvent-dependent equilibrium between heteroleptic (MLX) and homoleptic (ML2) species. At first, fluorescent MLX complex forms rapidly, but its subsequent reaction with excess ligand (L) and/or disproportionation leads to the formation of ML2, which precipitates out of the solution, thereby shifting the equilibrium. In coordinating solvents, where the solubilities of inorganic Zn salts, MLX and ML2 are high (e.g. in pyridine), the equilibrium can be shifted back to MLX by increasing the concentration of Zn2+. Notably, such equilibria are not common for metallodipyrrins, which typically exist as stable homoleptic adducts, notwithstanding that some heteroleptic complexes have been reported in the literature.16,17,18 The higher lability of our dipyrrinates could be a result of the steric and/or electronic effects of 2,2′-alkoxycarbonyl groups,19 which may weaken the coordination bonds and facilitate exchange reactions.
The direct evidence that heteroleptic BDP complexes are highly fluorescent was obtained by synthesizing a Zn dipyrrin-β-diketonate by way of a ligand exchange reaction. Treatment of 2a with the stoichiometric amount of Zn(acac)2 in acetone gave the desired Zn(acac)-2a, which readily precipitated from the reaction mixture. As expected, Zn(acac)-2a showed absorption features similar to those of BODIPY and bright red fluorescence (φfl=0.7 in THF) (S35).
The profound differences in the optical spectra of mono- and bis- complexes and complete on/off switching of fluorescence suggest presence of a strong exciton coupling effect in ML2.9 Exciton coupling has been observed in various chromophore dimers, including dimers of BODIPY.20 ML2 molecule may be viewed a “dimer” of two independent dipyrrin units, held close to one-another by the metal ion. In such a dimer, the two transition dipoles, oriented in the individual dipyrrins between the isoindolic residues (μ1 and μ2, Fig. 2c),17a can interact, resulting in a pair of non-degenerate states, shifted up and down relative to the transition of the parent ML complex (Fig. 2a, inset). The magnitude of the coupling and the intensities of the corresponding bands depend on the strengths of the transition dipoles and on their mutual orientation. In particular, non-planar orientation (in different planes at an angle to each other) may produce a splitting, in which the blue-shifted transition is allowed while the red-shifted one is forbidden.9
The experimental spectra of MLX and ML2 (Fig. 2a) appear to fit well the exciton coupling model. The peaks at 599 and 669 nm, obtained by fitting the absorption spectrum of ML2 with a pair of Lorentzians (S35), are shifted by 1118 cm−1 up and 629 cm−1 down relative to the principal band of the parent MLX complex (λmax=642 nm). The asymmetry is likely to be caused by the difference in the vibrational couplings and/or different solvation of the mono- and bis- complexes. The ratio of the oscillator strengths (ML2 vs MLX) was found to be 1.92, which is very close to the predicted by the theory (2.0).9 Fast non-radiative relaxation from the upper to the lower exciton state and slow emission from the latter, consistent with the low oscillator strength of the corresponding excitation, make the intersystem crossing and internal conversion the most likely causes for the loss of fluorescence in ML2. In toluene at 22°C, ML2 exhibits broad emission (λmax=785 nm) with negligible quantum yield φfl<0.01 and average lifetime of about 1.6 ns (S36).
Strong coupling of the transition dipoles suggests that the mutual orientation of the dipyrrin units in ML2 deviates from the strictly orthogonal (D2h symmetry). The X-ray crystal structure (CCDC # 749261) of ML2 Zn-2e (Fig. 3) fully corroborates this assumption. The angle between the mean-square planes of the dipyrrins in Zn-2e is only 64.5°, which is lower than found in the majority of known Zn dipyrrin complexes.21 Similarly “flattened” structures have been observed in Zn azadipyrrins (63–39°)22 and Zn α-methoxydipyrrin (54.7°).23 Also noteworthy is quite significant ruffling of the dipyrrin ligands, which resembles distortions of some non-planar porphyrins.24
Figure 3.

X-ray crystal structure (ORTEP) of homoleptic Zn-2e complex. Hydrogen atoms are omitted for clarity. Thermal ellipsoids are drawn at the 50% probability level.
Notably, the computed structure (DFT/B3LYP/6-31G(d)) of homoleptic Zn-2e (Fig. 2c) was also found non-orthogonal, although the angle between the mean square planes was somewhat larger (77°). This suggests that the distortion from the orthogonal geometry is not induced by the crystal packing forces, but is an intrinsic property of the ML2 molecule, which is flattened due to the propensity of the ligand π-systems to align in co-planar fashion.22 Non-orthogonal geometry may also facilitate interactions between the metal and the proximate carbonyl oxygens, which have been identified in some other homoleptic dipyrrin complexes.19b Although the Zn-O distances in Zn-2e are quite large (dZn-O=2.8–2.9Å vs dZn-N=2.0Å), carbonyl groups still could be implicated in the overall ligand-metal bond stabilization.19
In conclusion, the developed method of synthesis of fluorogenic π-extended dipyrrins allows tuning of their optical bands across the entire red/near infrared optical spectrum. Metal complexes of π-extended metallodipyrrins are strongly fluorescent, and their fluorescence can be switched on and off by changing the mode of metal coordination. The molecular exciton theory9 provides a new insight into the photophysics of metallodipyrrins, suggesting rational pathways to fluorogenic dipyrrin-based chelators. These results open up possibilities for engaging metallodipyrrins in a variety of applications, including construction of luminescent networks, electrooptical materials, biomedical imaging and sensing.
Supplementary Material
Acknowledgments
This work was supported by the grant 10-03-01122a from RFBR and the grant EB007279 from the NIH USA. SAV is grateful to Prof. R. M. Hochstrasser and Dr. L. E. Sinks for invaluable discussions.
Footnotes
Supporting Information Available. Synthetic procedures, spectroscopic data, fluorescence lifetime data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Wood TE, Thompson A. Chem Rev. 2007;107:1831. doi: 10.1021/cr050052c. [DOI] [PubMed] [Google Scholar]; (b) Ulrich G, Ziessel R, Harriman A. Angew Chem Int Ed. 2008;47:1184. doi: 10.1002/anie.200702070. [DOI] [PubMed] [Google Scholar]; (c) Benniston AC, Copley G. Phys Chem Chem Phys. 2009;11:4124. doi: 10.1039/b901383k. [DOI] [PubMed] [Google Scholar]; (d) Loudet A, Burgess K. Chem Rev. 2007;107:4891. doi: 10.1021/cr078381n. [DOI] [PubMed] [Google Scholar]
- 2.Fischer H. Ber Dtsch Ges. 1924;57:610. [Google Scholar]
- 3.Bruckner C, Karunaratne V, Rettig SJ, Dolphin D. Can J Chem. 1996;74:2182. [Google Scholar]
- 4.Yu LH, Muthukumaran K, Sazanovich IV, Kirmaier C, Hindin E, Diers JR, Boyle PD, Bocian DF, Holten D, Lindsey JS. Inorg Chem. 2003;42:6629. doi: 10.1021/ic034559m. and references therein. [DOI] [PubMed] [Google Scholar]
- 5.(a) Zhang YJ, Thompson A, Rettig SJ, Dolphin D. J Amer Chem Soc. 1998;120:13537. [Google Scholar]; (b) Thompson A, Rettig SJ, Dolphin D. Chem Commun. 1999:631. [Google Scholar]; (c) Thompson A, Dolphin D. J Org Chem. 2000;65:7870. doi: 10.1021/jo000886p. [DOI] [PubMed] [Google Scholar]; (d) Thompson A, Dolphin D. Org Lett. 2000;2:1315. doi: 10.1021/ol000053l. [DOI] [PubMed] [Google Scholar]
- 6.(a) Sazanovich IV, Kirmaier C, Hindin E, Yu LH, Bocian DF, Lindsey JS, Holten D. J Amer Chem Soc. 2004;126:2664. doi: 10.1021/ja038763k. [DOI] [PubMed] [Google Scholar]; (b) Thoi VS, Stork JR, Magde D, Cohen SM. Inorg Chem. 2006;45:10688. doi: 10.1021/ic061581h. [DOI] [PubMed] [Google Scholar]; (c) Sutton JM, Rogerson E, Wilson CJ, Sparke AE, Archibald SJ, Boyle RW. Chem Commun. 2004:1328. doi: 10.1039/b404147j. [DOI] [PubMed] [Google Scholar]; (d) Maeda H. J Incl Phenom Macrocycl Chem. 2009;64:193. [Google Scholar]
- 7.Ikeda C, Ueda S, Nabeshima T. Chem Commun. 2009:2544. doi: 10.1039/b820141b. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi J, Kushida T, Kawashima T. J Amer Chem Soc. 2009;131:10836. doi: 10.1021/ja904470m. [DOI] [PubMed] [Google Scholar]
- 9.(a) Kasha M, Rawls HR, El-Bayoumi MA. Pur Appl Chem. 1965;11:371. [Google Scholar]; (b) Kasha M. Physical Processes in Radiation Biology. Academic Press; New York: 1964. p. 17. [Google Scholar]
- 10.(a) Finikova OS, Aleshchenkov SE, Briñas RP, Cheprakov AV, Carroll PJ, Vinogradov SA. J Org Chem. 2005;70:4617. doi: 10.1021/jo047741t. [DOI] [PubMed] [Google Scholar]; (b) Filatov MA, Cheprakov AV, Beletskaya IP. Eur J Org Chem. 2007:3468. [Google Scholar]; (c) Filatov MA, Lebedev AY, Vinogradov SA, Cheprakov AV. J Org Chem. 2008;73:4175. doi: 10.1021/jo800509k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Cheprakov AV, Filatov MA. J Porph Phthalocyanines. 2009;13:291. [Google Scholar]
- 11.Fischer M, Georges J. Chem Phys Lett. 1996;260:115. [Google Scholar]
- 12.Shen Z, Rohr H, Rurack K, Uno H, Spieles M, Schulz B, Reck G, Ono N. Chem Eur J. 2004;10:4853. doi: 10.1002/chem.200400173. [DOI] [PubMed] [Google Scholar]
- 13.Donyagina VF, Shimizu S, Kobayashi N, Lukyanets EA. Tetrahedron Lett. 2008;49:6152. [Google Scholar]
- 14.Strickler SJ, Berg RA. J Chem Phys. 1962;37:814. [Google Scholar]
- 15.Lebedev AY, Filatov MA, Cheprakov AV, Vinogradov SA. J Phys Chem A. 2008;112:7723. doi: 10.1021/jp8043626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.(a) Jaumà A, Farrera JA, Ribó JM. Monatshefte Fur Chemie. 1996;127:927. [Google Scholar]; (b) Amiri A, Comeau IM, Thompson A. J Heterocycl Chem. 2006;43:431. [Google Scholar]; (c) Cipot-Wechsler J, Ali AAS, Chapman EE, Cameron TS, Thompson A. Inorg Chem. 2007;46:10947. doi: 10.1021/ic701369h. [DOI] [PubMed] [Google Scholar]; (d) Yadav M, Singh AK, Pandey DS. Organometallics. 2009;28:4713. [Google Scholar]
- 17.(a) Zakharova SP, Rumyantsev EV, Antina EV. Russ J Coord Chem. 2005;31:849. [Google Scholar]; (b) Rumyantsev EV, Kolpakov IE, Marfin YS, Antina EV. Russ J Gen Chem. 2009;79:482. [Google Scholar]
- 18.Teets TS, Partyka DV, Esswein AJ, Updegraff JB, Zeller M, Hunter AD, Gray TG. Inorg Chem. 2007;46:6218. doi: 10.1021/ic700776t. [DOI] [PubMed] [Google Scholar]
- 19.(a) Motekaitis RJ, Martell AE. Inorg Chem. 1970;9:1832. [Google Scholar]; (b) Clarke ET, Squattrito PJ, Rudolf PR, Motekaitis RJ, Martell AE, Clearfield A. Inorg Chim Acta. 1989;166:221. [Google Scholar]
- 20.(a) Bergstrom F, Mikhalyov I, Hagglof P, Wortmann R, Ny T, Johansson LBA. J Amer Chem Soc. 2002;124:196. doi: 10.1021/ja010983f. [DOI] [PubMed] [Google Scholar]; (b) Tleugabulova D, Zhang Z, Brennan JD. J Phys Chem B. 2002;106:13133. [Google Scholar]
- 21.Salazar-Mendoza D, Baudron SA, Hosseini MW. Inorg Chem. 2008;47:766. doi: 10.1021/ic701949k. [DOI] [PubMed] [Google Scholar]
- 22.Teets TS, Partyka DV, Updegraff JB, Gray TG. Inorg Chem. 2008;47:2338. doi: 10.1021/ic701190g. [DOI] [PubMed] [Google Scholar]
- 23.Halper SR, Stork JR, Cohen SM. Dalton Trans. 2007:1067. doi: 10.1039/b615801c. [DOI] [PubMed] [Google Scholar]
- 24.Senge MO. In: The Porphyrin Handbook. Kadish KM, Smith KM, Guilard R, editors. Ch 6. Academic Press; New York: 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.