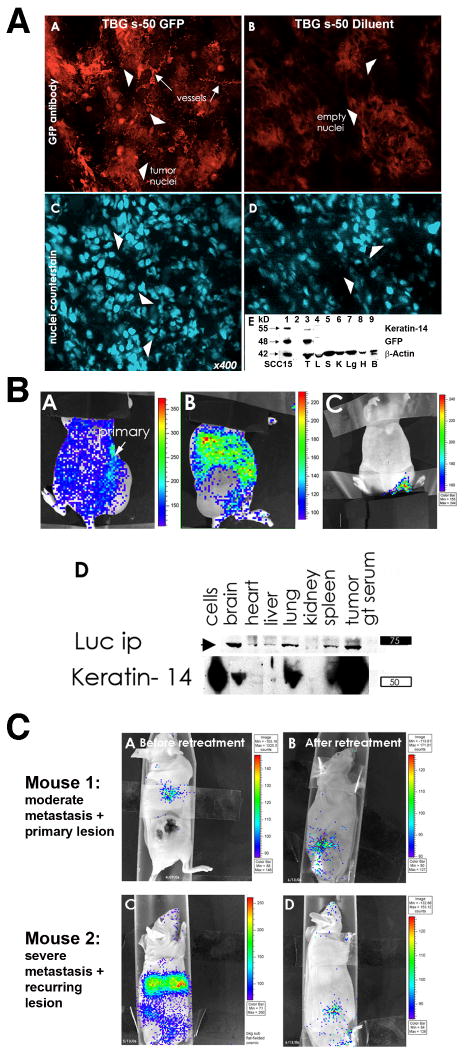
Fig. 1. TBG s-50 nanocapsule tumor-homing and biodistribution in xenograft mice following systemic administration.
A. TBG s-50 nanocapsules target tumor cells and tumor-derived microvessels following systemic distribution: Panels A-D - Tumor cryosections from mice bearing SCC-15 tongue carcinoma xenografts were immunodetected for nuclear GFP signal 3 weeks following i.p. administration at 0.2 mg/kg of TBG s-50 nanocapsules bearing nuclear-localized GFP plasmid (Panel A) or diluent (Panel B). Positioning of cell nuclei in tumors is depicted by bisbenzamide counterstain (Panels C,D). GFP immunosignal was located in the tumor-derived microvasculature (arrows, Panel A) and clusters of tumor cells (arrowheads, Panel A). Note the lack of nuclear GFP staining in the tumor sections from the TBG s-50 diluent injected mouse. Panel E - Immunoblot analysis of tumor and tissue lysates from mice treated with TBG s-50 nuclear GFP or diluent nanocapsules confirms microscopy by another method (only results for GFP treated mice shown). Lysates were subjected to electrophoresis on a 4-12% gradient gel and immunodetected for keratin-14 to identify tumor cells (Panel E-top line), GFP fusion protein (Panel E, middle line), and β-actin as a loading control (Panel E, lower line). The lanes contain the following samples: 1) GFP transfected SCC-15 cell line as positive control, 2) MW marker, 3) tumor, 4) liver, 5) spleen, 6) kidney, 7) lung, 8) heart and 9) brain.
B. TBG s-50 nanocapsules deliver nucleic acid cargo specifically to tumor metastases. Panels A-D - A mouse with a large UM-11b laryngeal carcinoma xenograft flank tumor was injected i.p. with TBG s-50 nanocapsules containing 12.5 μg of luciferase plasmid and imaged using the Xenogen method 5 days later to allow for development of luciferase gene expression (Panels A,B). A control mouse, first injected with TBG s-50 nanocapsules bearing trehalose, was injected after 5 days with luciferin substrate and imaged together with the luciferase mouse for background control and comparison (Panel C). Luciferase signal was apparent in the viable rim of the cytolytic tumor mass (Panel A) and throughout the lung and abdominal region (Panel B). Large peritoneal metastases were encountered upon necropsy, but not assayed further. Panel D - Tissues from the metastases-burdened mouse were immunoprecipitated for luciferase, and the immunoprecipitates were analyzed by immunoblot along with lysate to correlate luciferase expression with the presence of keratin-14, a marker for head neck cancer. Substantial keratin-14 signal was co-present with luciferase protein signal in brain, lung, spleen and tumor. The lane labeled “cells” represents UM-11b cells used for xenograft injection.
C. Existing metastases are cleared by TBG s-50 anti-CK2 nanocapsules in mice at ng/kg dosing. Panels A,C - Mice with SCC-15 tongue carcinoma xenografts were assayed for tumor burden 6 months following docetaxel/cisplatin treatment by the Xenogen method following i.p. administration of 12.5 μg of TBG s-50 luciferase plasmid. Panels B,D - Mice were administered additional tumor-targeted TBG s-50 luciferase plasmid nanocapsules and reimaged 4-6 weeks later following continual TBG s-50 anti-CK2 oligonucleotide nanocapsule treatment. The AB panel series was imaged after 6 weeks of 100 ng/kg q2d i.p. dosing together with topical 3 μg/ml TBG s-50 anti-CK2 oligonucleotide nanocapsule treatment for the last 2 weeks. The CD panel series was reimaged following 4 weeks of 100 ng/kg q2d i.p. treatment with TBG s-50 anti-CK2 oligonucleotide nanocapsules. Following reimaging, mice were treated with surgery (mouse in Panel B) or not (mouse in Panel D) and combination low-dose metronomic chemotherapy (20 μg/kg cisplatin, 1/1000 of murine MTD) with increasing doses of TBG s-50 anti-CK2 oligonucleotide nanocapsules before cessation of treatment. Mice were euthanized six months later at two years of age with no evidence of recurrence by imaging analysis.