Table 2. Structure and Binding Affinity of the Extended Series of Styryl and Alkoxy Bicyclononanes.
The ERα and ERβ binding affinity of both the styryl and alkoxy bicyclononane series was determined and expressed as outlined in the legend of Table 1.
| N [number of atoms: phenyl-carboxyl] | 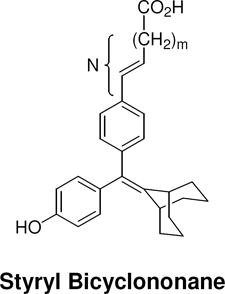 |
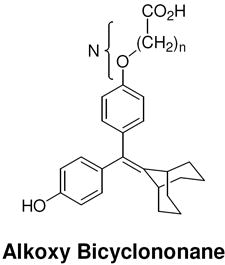 |
||||||
|---|---|---|---|---|---|---|---|---|
| N | lignad | RBAa ERα |
RBAa ERβ |
β/αb | lignad | RBAa ERα |
RBAa ERβ |
β/αb |
| 2 |
17c (m = 0) |
17.7 ± 4 | 25.9 ± 5 | 1.5 |
15c (n = 1) |
3.6 ± 1 | 3.1 ± 0.3 | 0.86 |
| 4 |
22 (m = 2) |
9.0 ± 2 | 23.1 ± 6 | 2.6 |
32 (n = 3) |
5.6 ± 1 | 10.5 ± 1 | 1.9 |
| 5 |
23 (m = 3) |
13.1 ± 2 | 24.6 ± 7 | 1.9 |
33c (n = 4) |
18.3 ± 3 | 38.3 ± 11 | 2.1 |
| 6 |
24c (m = 4) |
38.5 ± 0.2 | 62.8 ± 14 | 1.6 |
34c (n = 5) |
52.0 ± 6 | 89.1 ± 9 | 1.7 |
| 7 |
25c (m = 5) |
87.4 ± 10 | 81.1 ± 14 | 0.93 |
35 (n = 6) |
70.4 ± 20 | 139 ± 0 | 2.0 |
| 11 | -- | -- | -- |
36c (n = 10) |
95.6 ± 10 | 63.9 ± 17 | 0.67 | |
Relative binding affinity (RBA) values are determined by competitive radiometric binding assays and are expressed as IC50[estradiol] / IC50[compound] × 100 (RBA, estradiol = 100). In these assays the Kd for estradiol is 0.2 nM for ERα and 0.5 nM for ERβ.
For each value, the β/α ratio is calculated such that the ratio is >1 for compounds having higher affinity on ERβ than on ERα.
Compounds selected for further studies are indicated by a bold underline.
