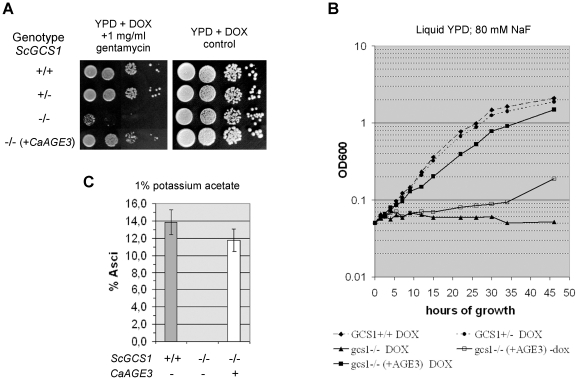
Figure 2. CaAGE3 complements defects of the yeast gcs1Δ mutant.
(A) The diploid S. cerevisiae strains BY4743 (wild type), ydl226c+/− (heterozygous for GCS1), ydl226c−/− (homozygous gcs1Δ) and UZ177 (homozygous gcs1Δ transformed with the centromeric plasmid pCaAge3-2 which carries the CaAGE3 ORF under control of the doxycycline-inducible Tet promoter) were grown for 48 hours on agar plates containing 50 µg/ml doxycycline (DOX) in the presence of gentamycin (A). The number of cells spotted onto the agar was about 20,000, 2,000, 200 or 20 (from left to right). (B) Exponentially pregrown cells (strains as in A) were inoculated into 80 mM sodium fluoride in YPD +50 µg/ml DOX and incubated at 30°C for 42 hours. Samples were taken after different time points and the OD600 measured. For control, each strain was also grown without DOX. Except for strain UZ177 the growth rate was independent of the presence of DOX (not shown). However, AGE3 induction in strain UZ177 by DOX results in complementation of the growth deficiency of gcs1Δ cells. This experiment was repeated and very similar growth behaviours were observed. (C) The wild-type, the homozygous gcs1Δ strain and UZ177 were induced for sporulation on agar plates containing 1% potassium acetate and 50 µg/ml DOX. The percentage of asci formation was determined after five days incubation at 30°C. This experiment was done in triplicate. Expression of CaAGE3 complements the complete inability of the gcs1Δ/Δ strain to form asci. Note that in general the sporulation efficiency of strains derived from BY4743 is low compared to other wild-type strains.