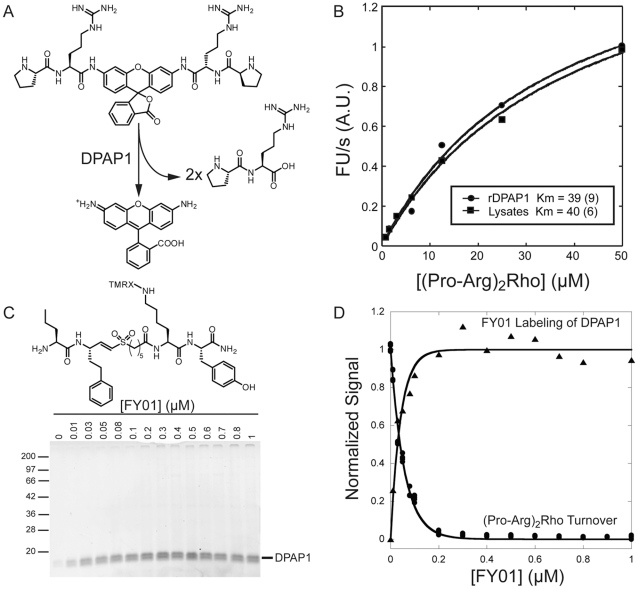
Figure 1. Use of an ABP to identify a DPAP1-selective substrate in parasite lysates.
A. Structure and reaction mechanism of the (Pro-Arg)2-Rho substrate. B. Measurement of (Pro-Arg)2-Rho apparent K m in trophozoite lysates (circles) and with recombinant DPAP1 (triangle). Turnover rates at increasing concentrations of substrate were fitted to a Michaelis-Menten equation as described in the methods section. C. Labeling of DPAP1 activity in parasite lysates with FY01. Trophozoite lysates were incubated for 1 h with increasing concentrations of FY01. Labeling was stopped by boiling the sample in SDS-PAGE loading buffer. DPAP1 activity was measured using a flatbed fluorescent scanner. D. DPAP1 labeling correlates with substrate turnover inhibition. An aliquot of the samples treated for 1 h with FY01 was diluted in assay buffer containing 10 µM of (Pro-Arg)2-Rho, and the initial turnover rate was measured in a 96-well plate (circles). This turnover rate is plotted with the labeling quantified in C.