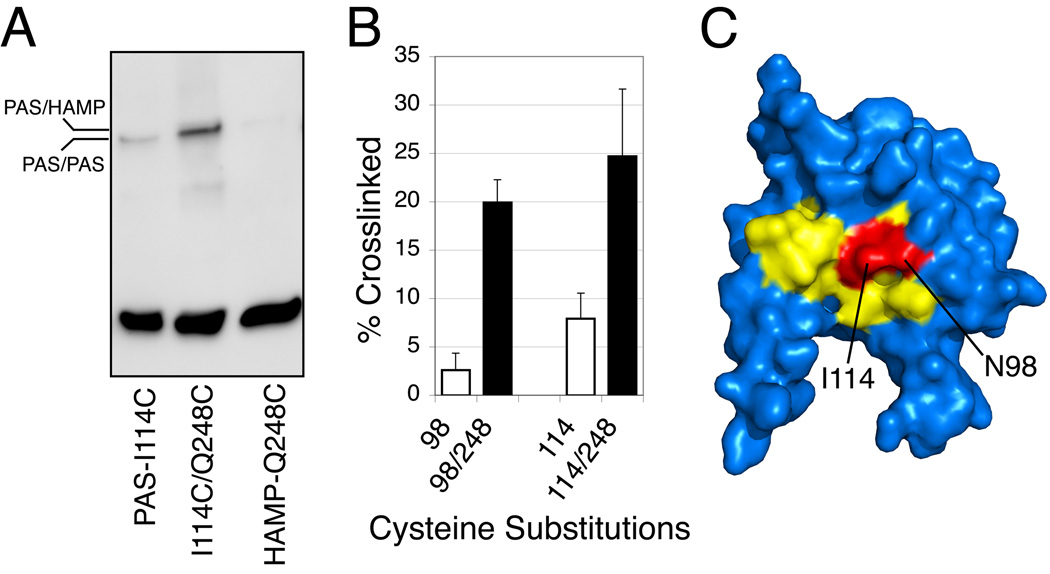
Fig. 6.
Crosslinking between residues in the Aer-PAS β-scaffolding and the Aer-HAMP AS-2 helix.
A. Western blot showing disulfide band formation between substitutions I114C in the PAS domain and Q248C in the HAMP AS-2 helix in response to the oxidant copper phenanthroline. Some crosslinking between cognate I114C residues occurred (lane 1) during maturation (it decreased in the presence of chloramphenicol; not shown), and it was absent in the Aer-I114C/Q248C double-replacement protein (lane 2). The extent of Q248C/Q248C crosslinking was approximately 1%. The mobility of the disulfide products indicated that PAS/HAMP contacts occurred between monomers rather than within monomers.
B. Extents of crosslinking between replacement Q248C in the HAMP AS-2 helix and either N98C or I114C in the β-scaffolding of the PAS domain. Whole cells were incubated with 600 µM copper phenanthroline for 20 min at 25° C (Taylor et al., 2007).
C. Residues I114 and N98 (red) lie within the signal-on cluster of substitutions (yellow) in the PAS β-scaffolding, supporting the hypothesis that conformational changes in this region are transmitted directly to the HAMP AS-2 helix.