Abstract
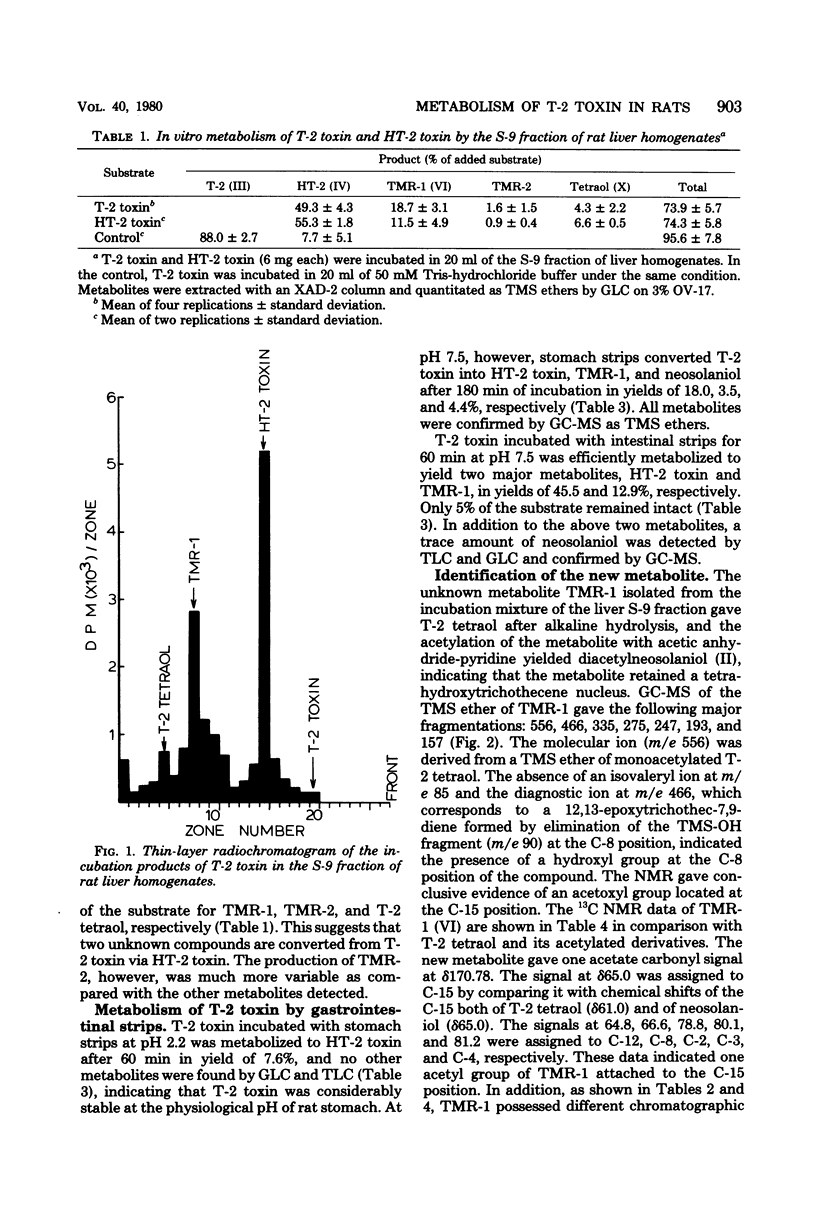
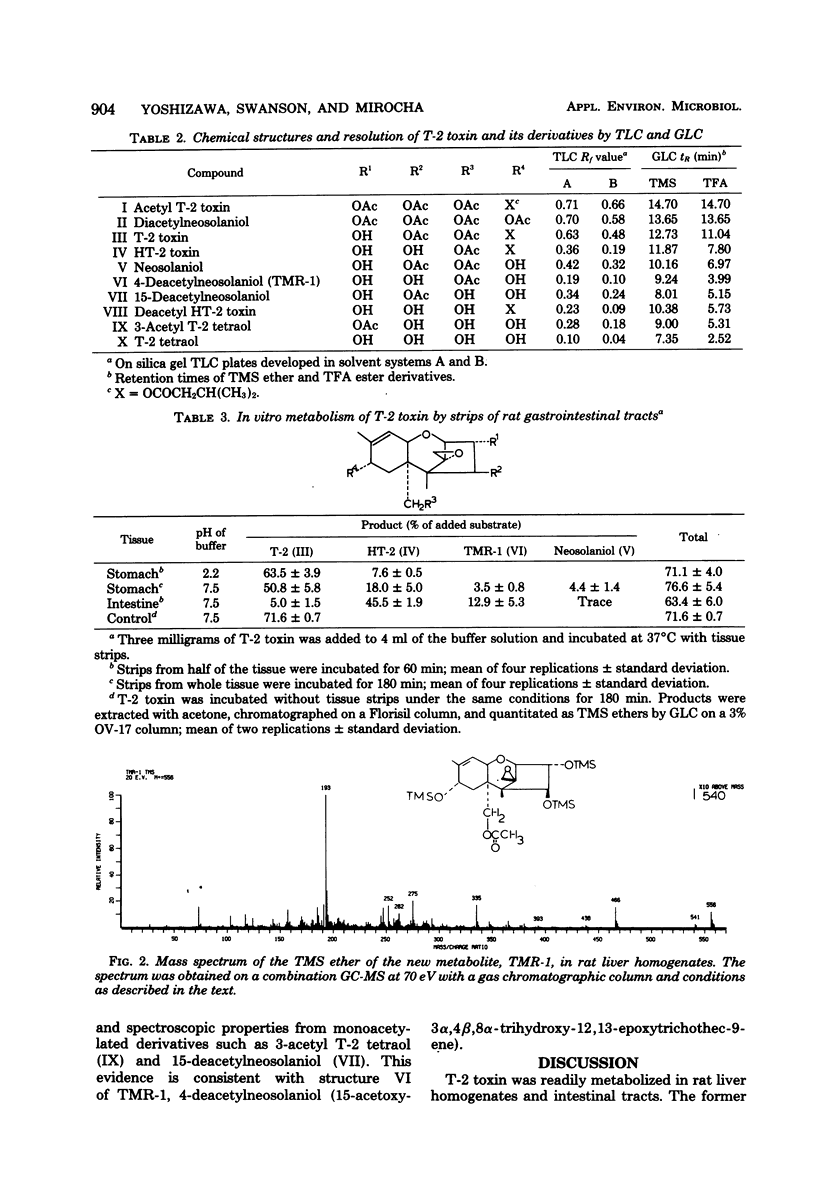
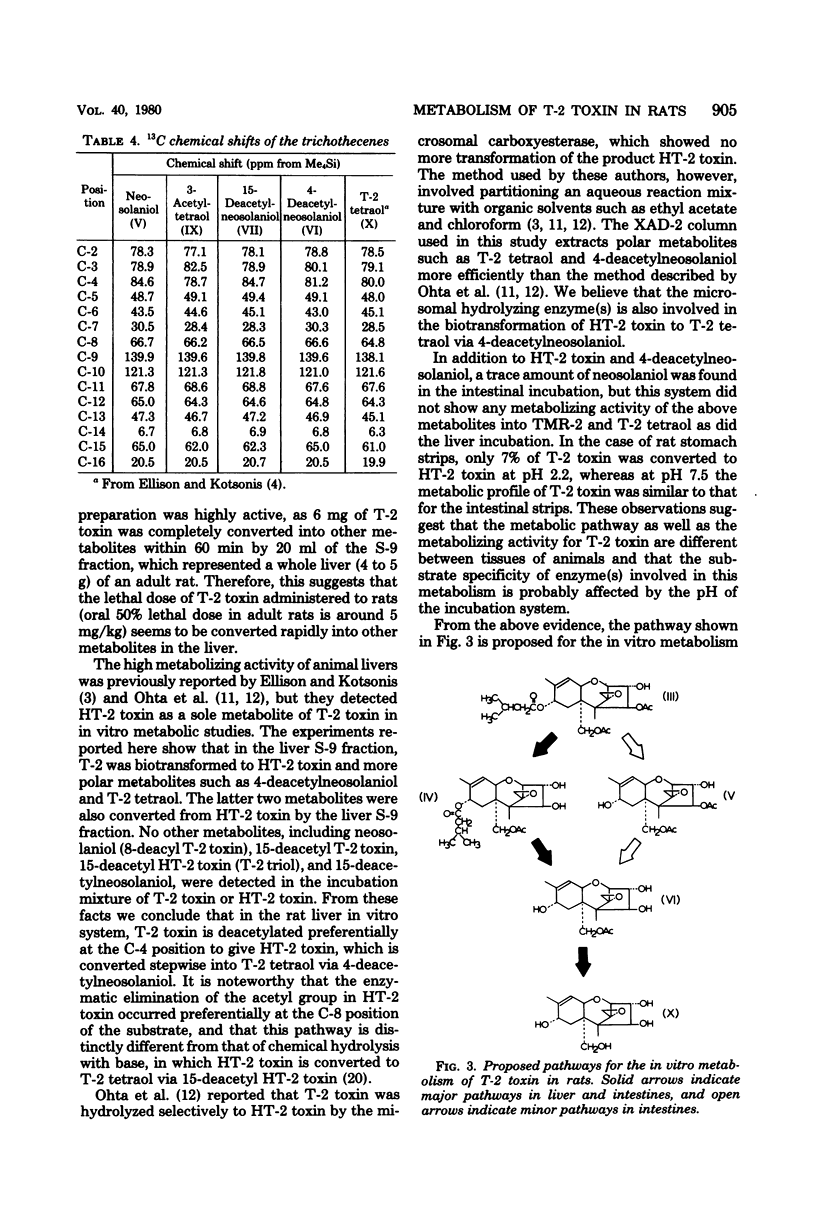
T-2 toxin was rapidly converted in the 9,000 X g supernatant fraction of rat liver homogenate into HT-2 toxin, T-2 tetraol, and two unknown metabolites designated as TMR-1 and TMR-2. TMR-1 was characterized as 4-deacetylneosolaniol (15-acetoxy-3 alpha, 4 beta, 8 alpha-trihydroxy-12,13-epoxytrichothec-9-ene) by spectroscopic analyses. Since the same metabolites were also obtained from HT-2 toxin used as substrate, it was concluded that T-2 toxin was hydrolyzed preferentially at the C-4 position to give HT-2 toxin, which was then metabolized to T-2 tetraol via 4-deacetylneosolaniol. In addition to HT-2 toxin, 4-deacetylneosolaniol and T-2 tetraol, a trace amount of neosolaniol was transformed from T-2 toxin by rat intestinal strips. In vitro metabolic pathways for T-2 toxin in rats are proposed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chi M. S., Robison T. S., Mirocha C. J., Reddy K. R. Acute toxicity of 12,13-epoxytrichothecenes in one-day-old broiler chicks. Appl Environ Microbiol. 1978 Apr;35(4):636–640. doi: 10.1128/aem.35.4.636-640.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison R. A., Kotsonis F. N. Carbon-13 nuclear magnetic resonance assignments in the trichothecene mycotoxins. J Org Chem. 1976 Feb 6;41(3):576–578. doi: 10.1021/jo00865a044. [DOI] [PubMed] [Google Scholar]
- Ellison R. A., Kotsonis F. N. In vitro metabolism of T-2 toxin. Appl Microbiol. 1974 Feb;27(2):423–424. doi: 10.1128/am.27.2.423-424.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal S., Biswas K., Srivastava R. S., Chakrabarti D. K., Chaudhary K. C. Toxic substances produced by Fusarium V: occurrence of zearalenone, diacetoxyscirpenol, and T-2 toxin in moldy corn infected with Fusarium moniliforme Sheld. J Pharm Sci. 1978 Dec;67(12):1768–1769. doi: 10.1002/jps.2600671238. [DOI] [PubMed] [Google Scholar]
- Hsu I. C., Smalley E. B., Strong F. M., Ribelin W. E. Identification of T-2 toxin in moldy corn associated with a lethal toxicosis in dairy cattle. Appl Microbiol. 1972 Nov;24(5):684–690. doi: 10.1128/am.24.5.684-690.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H., Ito T., Ueno Y. Toxicological approaches to the metabolities of fusaria. XII. Fate and distribution of T-2 toxin in mice. Jpn J Exp Med. 1978 Oct;48(5):393–399. [PubMed] [Google Scholar]
- Mirocha C. J., Pathre S. V., Schauerhamer B., Christensen C. M. Natural occurrence of Fusarium toxins in feedstuff. Appl Environ Microbiol. 1976 Oct;32(4):553–556. doi: 10.1128/aem.32.4.553-556.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirocha C. J., Schauerhamer B., Christensen C. M., Kommedahl T. Zearalenone, deoxynivalenol, and T-2 toxin associated with stalk rot in corn. Appl Environ Microbiol. 1979 Sep;38(3):557–558. doi: 10.1128/aem.38.3.557-558.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta M., Ishii K., Ueno Y. Metabolism of trichothecene mycotoxins. I. Microsomal deacetylation of T-2 toxin in animal tissues. J Biochem. 1977 Dec;82(6):1591–1598. doi: 10.1093/oxfordjournals.jbchem.a131854. [DOI] [PubMed] [Google Scholar]
- Ohta M., Matsumoto H., Ishii K., Ueno Y. Metabolism of trichothecene mycotoxins. II. Substrate specificity of microsomal deacetylation of trichothecenes. J Biochem. 1978 Sep;84(3):697–706. doi: 10.1093/oxfordjournals.jbchem.a132175. [DOI] [PubMed] [Google Scholar]
- Puls R., Greenway J. A. Fusariotoxicosis from barley in British Columbia. II. Analysis and toxicity of syspected barley. Can J Comp Med. 1976 Jan;40(1):16–19. [PMC free article] [PubMed] [Google Scholar]
- Rukmini C., Bhat R. V. Occurrence of T-2 toxin in Fusarium-infested sorghum from India. J Agric Food Chem. 1978 May-Jun;26(3):647–649. doi: 10.1021/jf60217a013. [DOI] [PubMed] [Google Scholar]
- Szathmary C. I., Mirocha C. J., Palyusik M., Pathre S. V. Identification of mycotoxins produced by species of Fusarium and Stachybotrys obtained from Eastern Europe. Appl Environ Microbiol. 1976 Oct;32(4):579–584. doi: 10.1128/aem.32.4.579-584.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno Y., Ishii K., Sakai K., Kanaeda S., Tsunoda H. Toxicological approaches to the metabolites of Fusaria. IV. Microbial survey on "bean-hulls poisoning of horses" with the isolation of toxic trichothecenes, neosolaniol and T-2 toxin of Fusarium solani M-1-1. Jpn J Exp Med. 1972 Jun;42(3):187–203. [PubMed] [Google Scholar]
- Ueno Y., Sato N., Ishii K., Sakai K., Enomoto M. Toxicological approaches to the metabolites of Fusaria. V. Neosolaniol, T-2 toxin and butenolide, toxic metabolites of Fusarium sporotrichioides NRRL 3510 and Fusarium poae 3287. Jpn J Exp Med. 1972 Oct;42(5):461–472. [PubMed] [Google Scholar]
- Wallace E. M., Pathre S. V., Mirocha C. J., Robison T. S., Fenton S. W. Synthesis of radiolabeled T-2 toxin. J Agric Food Chem. 1977 Jul-Aug;25(4):836–838. doi: 10.1021/jf60212a058. [DOI] [PubMed] [Google Scholar]
- Wei R., Strong F. M., Smalley E. B., Schnoes H. K. Chemical interconversion of T-2 and HT-2 toxins and related compounds. Biochem Biophys Res Commun. 1971 Oct 15;45(2):396–401. doi: 10.1016/0006-291x(71)90832-1. [DOI] [PubMed] [Google Scholar]
- Yoshizawa T., Swanson S. P., Mirocha C. J. T-2 metabolites in the excreta of broiler chickens administered 3H-labeled T-2 toxin. Appl Environ Microbiol. 1980 Jun;39(6):1172–1177. doi: 10.1128/aem.39.6.1172-1177.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


