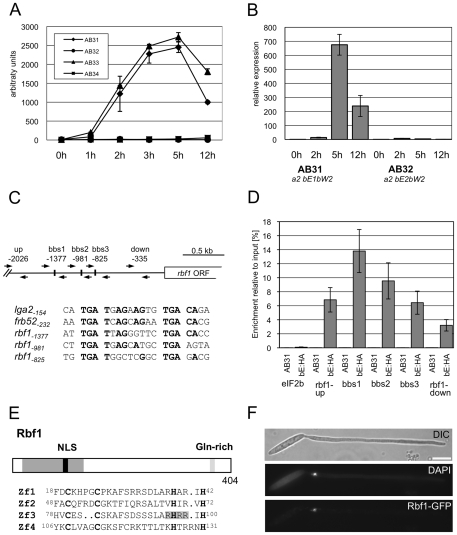
Figure 2. Structure and expression of Rbf1.
(A) Microarray analysis of b-dependent rbf1 expression after induction of compatible (AB31 and AB33) and incompatible (AB32 and AB34) combinations of bE and bW. Shown are the mean expression values of two biological replicates and the standard deviation (SD) (B) qRT-PCR analysis of rbf1 expression after induction of compatible (AB31) and incompatible (AB32) combinations of bE and bW. Samples were taken at the time-points indicated. qRT-PCR analysis was performed using the constitutively expressed ppi gene (um03726) for normalization. Expression was calculated relative to the lowest expression value. Shown are mean values of two technical replicates. (C) Overview of primer binding sites in the rbf1-promoter used for qChIP experiments and alignment of three putative b-binding sites (bbs) in the rbf1 promoter region to the bbs of lga2 and frb52 [10], [11]. Nucleotide positions indicated are relative to the start codon. Nucleotides identical to the bbs in lga2 [10] and frb52 [11] are in bold. (D) qChIP analysis of bE1 binding to the rbf1-promoter in strains AB31 and AB31bE1:3xHA 5h after induction of the bE1/bW2-heterodimer. AB31bE1:3xHA harbours a HA-tagged bE1 protein used for immunoprecipitation with anti-HA-antibody. Numbers give the enrichment in % of the input-DNA of the PCR amplicons in DNA co-immunoprecipitated with HA-antibody. No significant enrichment was observed in control strain AB31. In AB31bE1:3xHA, the PCR-amplicon spanning bbs1 (bbs−1377) is significantly enriched (t-test) when compared to the amplicon spanning a control region (−2026) (p = 5.71 10−5). As additional control, a region from the eIF2b gene (um04869) was used. Given are the mean values of three technical replicates of three independent experiments each, and the standard deviation (SD). (E) Structure of the Rbf1 protein. The potential C2H2 zinc finger domain (aa 18 to 131) and a putative NLS (RHRR) (aa 95 to 98) within this domain are marked in dark grey and black, a glutamine rich sequence (aa 365 to 373) is marked in grey. The alignment shows the four C2H2 zinc finger domains; the conserved cysteine and histidine residues are in bold. (F) Subcellular localization of the Rbf1-3xeGFP fusion protein. Strain AB31rbf1:3eGFP (UMS63) was induced in CM medium supplemented with 1% arabinose (CMA) for eight hours. The functional Rbf1-3xeGFP fusion protein localizes to the nucleus. Cells were stained with DAPI to visualize nuclei. Scale bar corresponds to 10 µm.