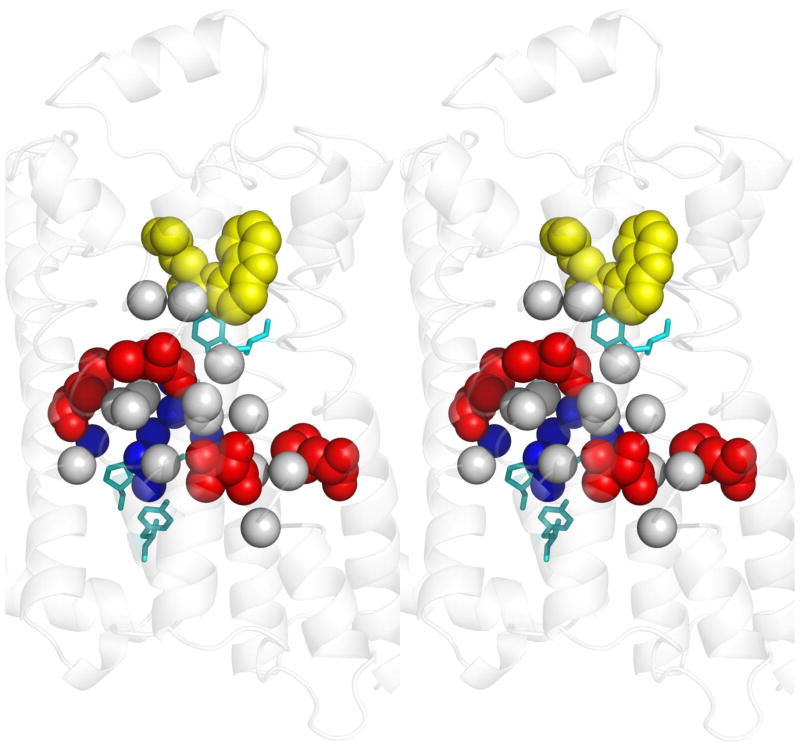
Figure 2. Determinants of allosteric specificity in psychoactive receptors.
A stereo view of the β2-adrenergic receptor structure bound to carazolol (yellow) (PDB:2rh1 [94]) provides a homology model for bioamine receptors, including the D2R and 5HT2AR dopamine and serotonin receptors. Four top-ranked amino acids (red), that are part of a putative allosteric pathway identified by ET, were swapped into D2R from their cognates in 5HT2AR and this conferred significant serotonin responsiveness to the mutants even though none had increased affinity to serotonin (red). Two of the four also had decreased dopamine responsiveness with either no change or with a paradoxical increase in dopamine affinity. Although distant from the ligand binding site, these residues are closely associated to other top-ranked ET residues in the putative allosteric pathway that are invariant between D2DR and 5HT2AR (Cα atoms of residues within 5 Å, gray). Together, these top-ranked residues link the toggle switch (top, cyan residue) and the NPxxY motif (lower cyan residues) that are generic GPCR mediators of activation; and they also surround a pocket of structural waters (blue spheres). Like rekeying the tumblers of a lock, the exchange of these residues shifted the sensitivity of the allosteric pathway from one ligand to another, independently of changes to binding affinity. These allosteric specificity determinants are consistent with the pharmacology of ligand-biased signaling [81].