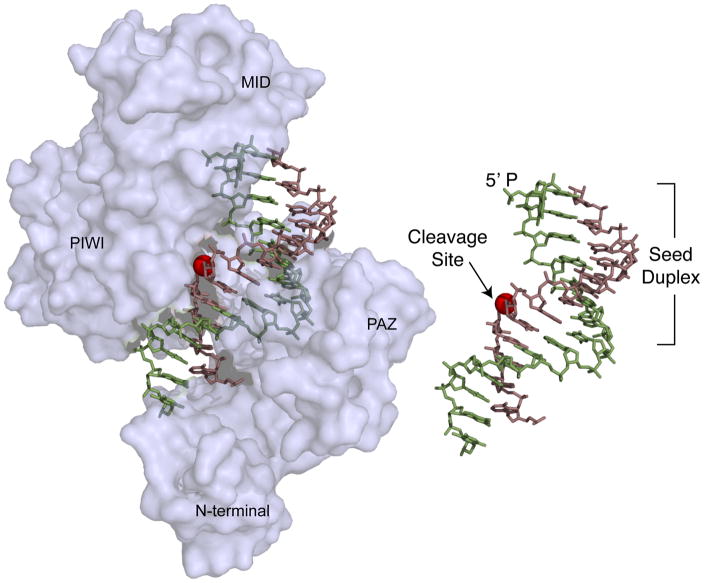
Figure 4.
Crystal structure of a Thermus thermophilus DNA-dependent RNA endonuclease related to eukaryotic Argonaute proteins [46]. The structure shows the protein bound to a DNA guide (green) and a target RNA (violet). The 5′ monophosphate end of the guide is anchored in a cleft between the MID and the PAZ domains. Nucleotides 2–8, which comprise the seed, are exposed on the surface of the protein complex and as such are positioned for substrate recognition. Pairing of the 3′-end of the guide with the target RNA aligns the scissile phosphate (red sphere) with the catalytic residues in the protein. The inset shows the guide target interaction in the absence of protein for clarity.