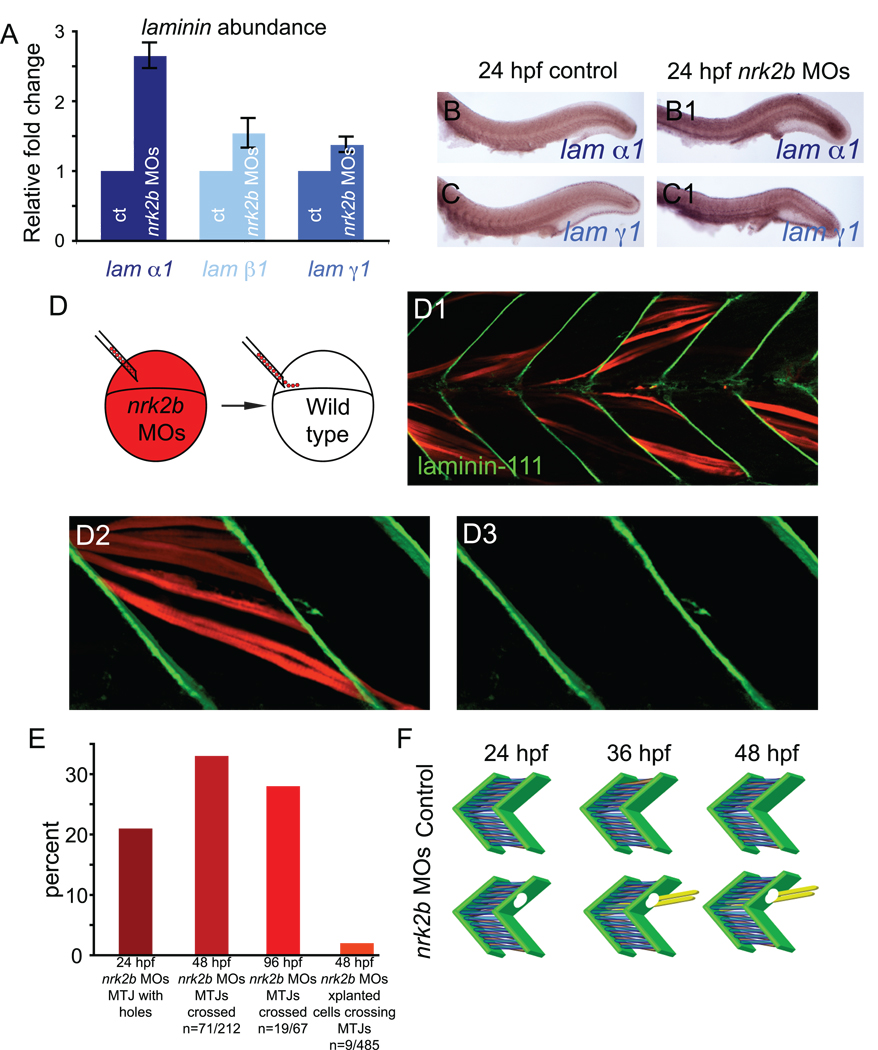
Fig. 5. Nrk2b is non-cell autonomously required for normal basement membrane polymerization.
(A) qRT-PCR for relative transcript abundance of the three laminin chains that comprise Laminin-111 in 24 hpf embryos. (B-C) Side mount, anterior left, dorsal top, 24 hpf. ISH for laminin transcripts in controls (lettered panels) and nrk2b morphants (numbered panels), (panels B) laminin α1, (panels C) laminin γ1. ISH and qRT-PCR suggest that Laminin-111 defects in nrk2b morphants are not due to a laminin transcription deficiency. (D) Model of dextran labeled nrk2b morphant cells being transplanted into a wild-type host at blastula stage. (D1-D3) Side mount, anterior left, dorsal top, 48 hpf, red cells are dextran labeled Nrk2b-deficient cells. Laminin-111 antibody staining (in green) is normal adjacent to Nrk2b-deficient cells (red) in a wild type background. This suggests that basement membrane polymerization is a phenomenon mediated by communities of cells and that Nrk2b is non-cell autonomously required for basement membrane polymerization. (E) Graph of the percentage of MTJs with holes in Laminin-111 or with boundary crossings over time. The observation that the percentage of MTJs with defects does not steadily increase over time suggests that boundary crossings are not due to age-dependent degeneration of the MTJ. Note that a very small percentage of Nrk2b-deficient cells cross MTJs in wild-type hosts suggesting that these cells do not actively degrade Laminin-111 and that Nrk2b functions non-cell autonomously in basement membrane development. (F) Model of MTJ basement membrane defects in nrk2b morphants leading to fibers crossing MTJs as time progresses.