Table 1.
Enantioselective decarboxylative enolate alkylation cascadea.
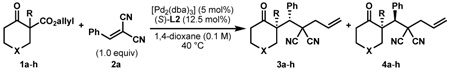 | |||||||
|---|---|---|---|---|---|---|---|
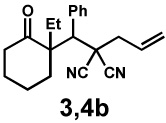
| Entry | Substrate | Product | Time | Yieldb | dr(3:4)c | ee(3) | ee(4) |
| 1 | 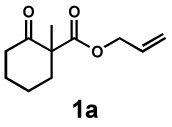 |
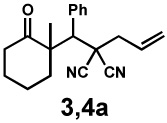 |
24 h | 99% | 1 : 6.1 | 77% | 87% |
| 2 | 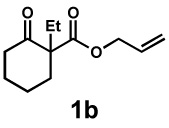 |
 |
48 h | 91% | 1 : 3.5 | 95% | 99% |
| 3d | 72 h | 88% | 1 : 3.4 | 88% | 97% | ||
| 4 | 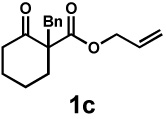 |
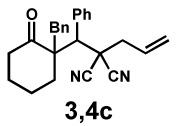 |
40 h | 49% | 1 : 1.9 | 85% | 88% |
| 5e | 65 h | 65% | 1 : 1.9 | 93% | 94% | ||
| 6 | 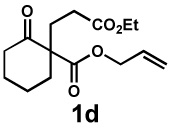 |
 |
24 h | 56% | 1 : 3.3 | 82% | 89% |
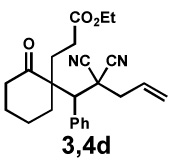
| 7f | 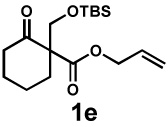 |
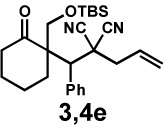 |
24 h | 56% | 1 : 1.3 | 69% | 70% |
| 8g | 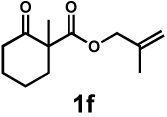 |
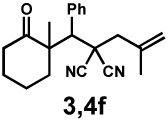 |
72 h | 57% | 1 : 2.4 | 75% | 81% |
| 9 | 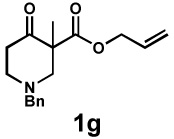 |
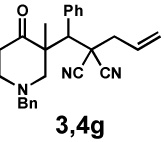 |
20 h | 97% | 1 : > 20 | - | 89% |
| 10 | 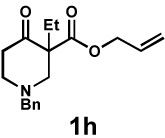 |
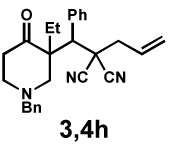 |
48 h | 99% | 1 : > 20 | 71% | 97% |
General reaction conditions: 1 (0.3–0.5 mmol), 2 (1.0 equiv), [Pd2(dba)3] (5 mol%), L2 (12.5 mol%), 1,4-dioxane (0.1 M), 40 °C.
Combined isolated yield.
Determined by 1H nmr.
Carried out on 1 mmol scale with 2.5 mol% [Pd2(dba)3] and 6.25 mol% L2.
Reaction performed at 23 °C.
Pd(pmdba)2 (10 mol%) was used as precatalyst.
Reaction performed at 60 °C.
