Table 2.
Carbodiimide Scope
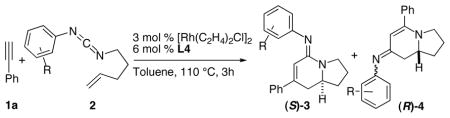 | ||||
|---|---|---|---|---|
| entry | R | 3:4b | yield (%) of 3c | ee (%) of 3d |
| 1 | p-OMe, 2b | 3.6: 1 | 70 | 94 |
| 2 | o-OMe, 2c | 3.8: 1 | 68 (62)e | 98 (96)e |
| 3 | m-Cl, 2d | 3.4: 1 | 67 | 97 |
| 4 | o-CF3, 2e | 9.4: 1 | 82 | 97 |
| 5f | p-CN, 2f | 9.5: 1 | 55 | 92 |
Conditions: 1 (2 equiv), 2 (0.16 mmol), Rh catalyst, L in PhMe at 110 °C.
Product selectivity (3: 4) is determined by 1H NMR of the unpurified reaction mixture.
Isolated yield.
Determined by HPLC analysis using a chiral stationary phase.
0.8 mmol scale of 2c, 1 mol% Rh catalyst and 2 mol% L4.
5 mol % Rh catalyst and 10 mol % L4.
