Table 3.
Terminal Alkyne Scope
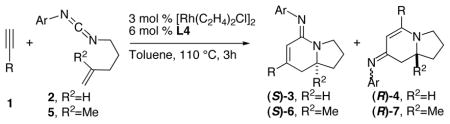 | |||||
|---|---|---|---|---|---|
| entry | R | carbodiimide | 3: 4 or 6: 7b | yield (%) of 3 or 6c | ee (%) of 3 or 6d,e |
| 1 | p-Br-C6H4, 1b | 2c | 16: 1 | 75 | 98 |
| 2 | m-F-C6H4, 1c | 2c | > 19: 1 | 77 | 99 |
| 3 | 3,5-F-C6H3, 1d | 2c | > 19: 1 | 66 | 99 |
| 4 | p-Ac-C6H4, 1e | 2c | > 19: 1 | 78 | 99 |
| 5 | p-CF3-C6H4, 1f | 2c | > 19: 1 | 68 | 96 |
| 6 | m-CN-C6H4, 1g | 2c | > 19: 1 | 62 | 94 |
| 7 | m-OMe-C6H4, 1h | 2c | 6.3: 1 | 69 | 99 |
| 8 | m-Me-C6H4, 1i | 2c | 3.4: 1 | 61 | 98 |
| 9 | “ | 2e | 8.3: 1 | 74 | 98 |
| 10 |
|
2c | 3.2: 1 | 58 | 98 |
| 11 | “ | 2e | 7.2: 1 | 79 | 97 |
| 12 | p-OMe-C6H4, 1k | 2c | 1: 2.8 | 20 (52)g | 99 |
| 13 | “ | 2e | 1: 1 | 37 (36)g | 96 |
| 14 | 3,5-F-C6H3, 1d | 5 | > 19: 1 | 79 | 98 |
| 15 | p-Ac-C6H4, 1e | 5 | 16: 1 | 74 | 99 |
| 16 | m-OMe-C6H4, 1h | 5 | 4.5: 1 | 66 | 96 |
| 17f | n-Hex, 1l | 2c | > 19: 1 | 74 | 91 |
| 18f | (CH2)4CO2Me, 1m | 2c | > 19: 1 | 68 | 92 |
| 19f | CH2CH2OTBS, 1n | 2c | > 19: 1 | 76 | 96 |
| 20f | (CH2)2CH2OTBS, 1o | 2c | > 19: 1 | 73 | 94 |
| 21f | (CH2)3CH2Cl, 1p | 2c | > 19: 1 | 60 | 88 |
| 22f | CH2CH2Ph, 1q | 2c | > 19: 1 | 70 | 92 |
Conditions: 1 (2 equiv), 2 (0.16 mmol), Rh catalyst, L in PhMe at 110 °C.
Product selectivity (3: 4) is determined by 1H NMR of the unpurified reaction mixture.
Isolated yield.
Determined by HPLC analysis using a chiral stationary phase.
Absolute configuration assigned by analogy to (S)-3bc (established by X-ray analysis).
5 mol % Rh catalyst and 10 mol % L4 employed.
Isolated yield of 4 (a 2:1 mixture of imine isomers).
