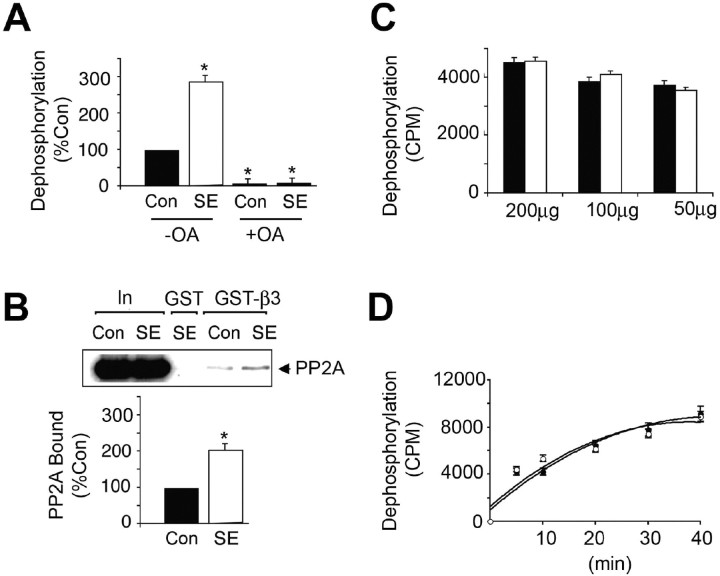
Figure 4.
Enhanced association of the GABAAR β3 subunit with PP2A in SE. A, Dephosphorylation of PNPP by hippocampal extracts. GST-β3 was exposed to lysates from control (filled bars) and SE (open bars) hippocampus, and the ability of bound material bound to dephosphorylated 14.2 μm PNPP in the absence (−) and presence (+) of 20 nm okadaic acid (OA) was measured. Data were then normalized to the level of dephosphorylation seen with control extracts (100%). B, Increased binding of PP2A to GST-β3 from SE extracts. Top, Material bound to GST-β3 or 10% of the input used for each immunoprecipitation (In) was immunoblotted with an antibody against the PP2A catalytic subunit. Bottom, The level of PP2A bound from control (filled bar) and SE (open bar) extracts was then determined, and values were normalized to those evident in control (100%). C, D, Dephosphorylation of 32P-GST-β3 by hippocampal extracts. Hippocampal lysates were prepared from control (filled bars and circles) and SE (open bars and circles). C, The ability of 200–50 μg of total detergent-soluble protein from these extracts to dephosphorylate 32P-GST-β3 was measured over a 10 min time course. D, The ability of 50 μg of total protein from control (filled circles) and SE (open circles) to dephosphorylate 32P-GST-β3 over a time course of 40 min was also examined. In all panels, asterisks indicate significant difference from control (p < 0.01; Student's t test; n = 3–4).