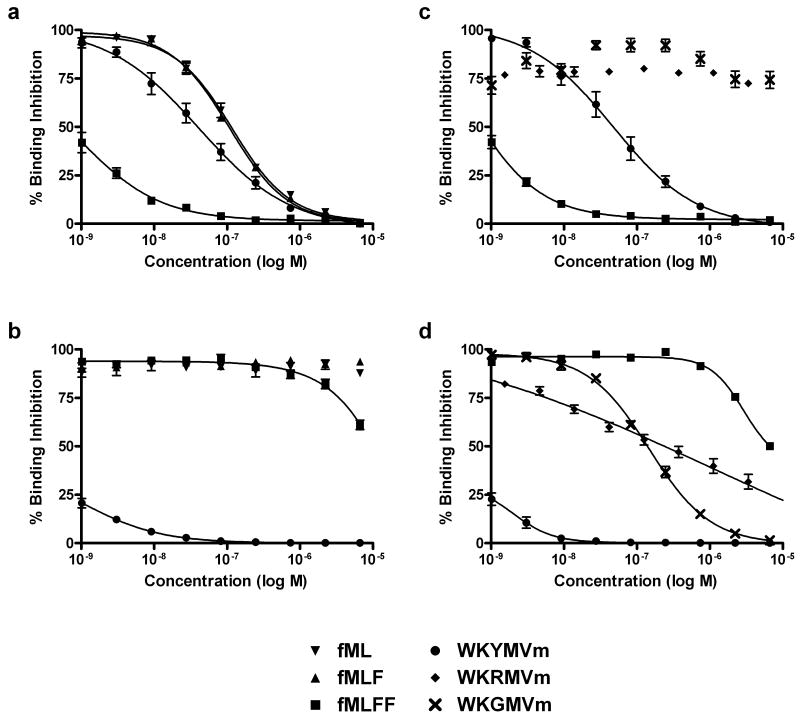
Figure 2.
Direct comparison of unlabeled FPR and FPRL1 peptide ligands through a competitive binding assay with the fluorescent WK(FL)YMVm probe. A: This plot shows the direct comparison of the FPR selective formylpeptide ligands (fML, fMLF, fMLFF) with the FPRL1 selective WKYMVm in FPR expressing U937 cells. The high-affinity fMLFF stands out with a 1.0 ± 0.6 nM EC50 while WKYMVm is only 43.8 ± 4.3 nM. The other ligands, fML and fMLF, had EC50 values in the 100 nM range. B: In FPRL1 expressing RBL cells the WKYMVm ligand shows high affinity (EC50 = 1.8 ± 0.3 nM), whereas the formyl peptides are all effectively nonbinding. C: The W-peptides WKRMVm and WKGMVm show no binding in FPR expressing U937 cells. D: Moderate binding of the W-peptides is demonstrated in FPRL1 expressing RBL cells at EC50 values of 704.8 ± 384.1 nM and 175.6 ± 40.6 nM for WKRMVm and WKGMVm, respectively.