Abstract
The ubiquitin proteasome system plays a role in regulating protein activity and is integral to the turnover of damaged and worn proteins. In this review, we discuss the recently described relationship between the ubiquitin proteasome system and the cardiac creatine kinase/phospho-creatine shuttle, an essential component of ATP generation and energy shuttling within the heart. The ubiquitin ligase muscle ring finger-1 (MuRF1) binds creatine kinase, leading to its ubiquitination and possible degradation. MuRF1 may also be integral in the regulation of creatine kinase activity in vivo. Since there is a close relationship between the cardiac creatine kinase/phospho-creatine shuttle activity and heart failure, these findings suggest that MuRF1’s role in protein quality control of creatine kinase may be vital to the regulation and maintenance of cardiac energetics to protect against heart failure.
Introduction
The demand for intracellular ATP is approximately 10,000 times greater than the actual amount of ATP present in cells (Ingwall 2009), resulting in the need for highly energetic cells like cardiomyocytes to regenerate ATP so as to maintain pump function. The regeneration of ATP is maintained, in part, by the oxidation of fatty acids and glucose. During cardiac stress, additional pathways are also utilized, including glycolysis and phosphotransferase systems mediated by creatine kinase (CK) and adenylate kinase. The phosphotransferase system also acts as a metabolic relay, transferring ATP between its site of production (mitochondria) and its site of utilization (e.g. sarcomere). The fact that the regulation of these systems changes in response to the available energy supply has been known for some time. However, our knowledge of the mechanism by which this regulation occurs is relatively sparse. In this review, we discuss recent studies identifying the role of the ubiquitin ligase muscle ring finger-1 (MuRF1) in the protein quality control of the phosphotransferase system mediated by CK. A clearer understanding of how these systems are regulated may offer insight into both the pathogenesis of and potential new therapies for heart failure.
The ubiquitin proteasome system (UPS) and protein quality control
Protein turnover and the degradation of specific proteins are essential for regulating every aspect of cellular function. In eukaryotic cells, the ubiquitin proteasome system (UPS) selectively tags proteins with covalently-linked ubiquitin for degradation by the proteasome (see Figure 1). The UPS serves two major roles: 1) regulating protein function; and 2) regulating protein quality control. UPS-mediated degradation is essential in regulating key cellular processes such as apoptosis and cell cycle (Pines 2006). Likewise, during periods of hypoxic stress within the cell, the UPS-regulates degradation of transcription factors such as HIF1α in an oxygen-dependent manner (Jaakkola et al. 2001). The UPS is also important for regulating protein quality control. The UPS recognizes and degrades misfolded proteins that accrue within the cell owing to the “wear and tear” that occurs during the normal homeostasis of cellular processes. Recently it has also been suggested that post-translational modification and damage may be a signal for protein degradation by the UPS. For example, mildly oxidized proteins are degraded by the proteasome (Dunlop et al. 2009), suggesting that oxidative changes to proteins, such as carbonylation, may be one signal that elicits the recognition by ubiquitin ligases to target specific proteins for destruction. Furthermore, evidence for UPS-involvement in the maintenance of the creatine kinase/phospho-creatine system has also recently been demonstrated.
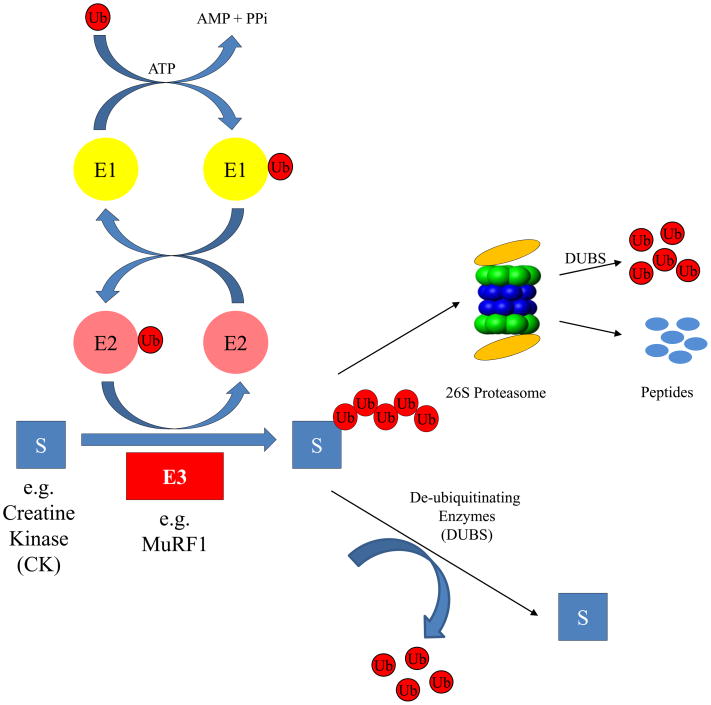
Figure 1.
The canonical Ubiquitin Proteasome System (UPS). Protein ubiquitination is driven by the action of 3 successive enzymes. The ubiquitin-activating enzyme (E1) activates ubiquitin in an ATP-dependent manner, adding the ubiquitin to the subsequent ubiquitin conjugating (E2) enzyme. Ubiquitin is then transferred from the ubiquitin-charged E2 to the substrate and/or the growing ubiquitin chain by the activity of a ubiquitin ligase (E3), which gives the system specificity. The multi-ubiquitin chain attached to the substrate is recognized by the 26S proteasome, which degrades the substrate and ubiquitin chain. The selective nature of the ubiquitin ligases is the focus of many investigations since it drives the specificity of the reaction. The canonical UPS degradation system shown here represents a skeletal schematic for a much more complex system, with ancillary factors participating in substrate recognition and a pathway involved in regulating protein activities by post-translational modifications that don’t always lead to degradation (Elsasser et al. 2004;Li et al. 2007;Richly et al. 2005).
The creatine kinase/phospho-creatine (CK/PCr) system
The CK/PCr is a buffering system essential for cells that have a high and fluctuating energy requirement, such as myocytes. It connects the site of ATP production (mitochondria) with those sites where energy is needed (for example the sarcomere; Figure 2). The CK enzyme transfers the N-phosphoryl group from phospho-creatine (PCr) to ADP to regenerate ATP (Figure 2A and 2B). There are four isoenzymes of CK, whose names are derived from their original source of isolation: CK-MM derived from muscle; CK-BB derived from brain; CK-MB found in the heart and mtCK derived from mitochondria. The CK-MM isoform exists within myofibrils as part of the structures localized to the M-band and is functionally coupled to myosin ATPase, allowing for the efficient maintenance of high ATP/ADP locally, stimulation of ATPase activity and assurance of proper contraction of the muscle (Ventura-Clapier et al. 1994).
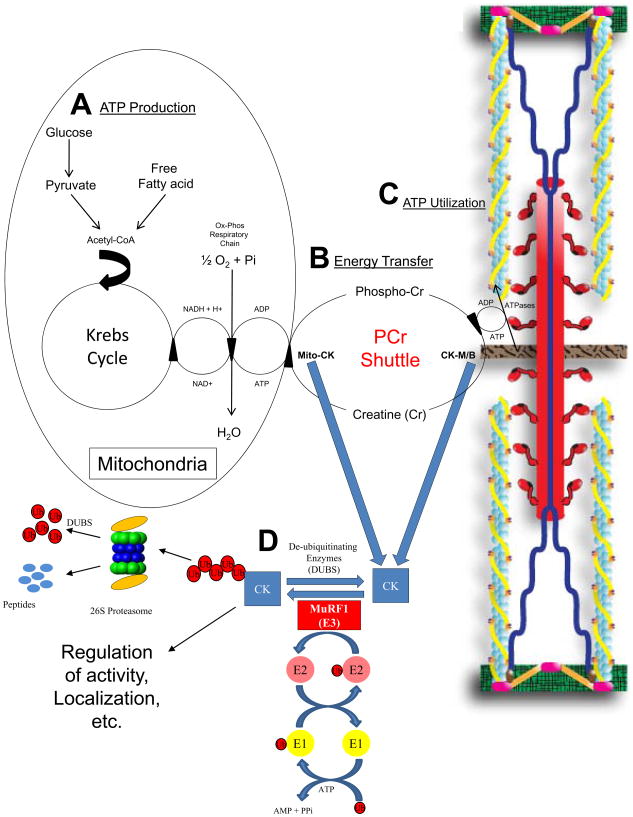
Figure 2.
MuRF1’s regulation of CK activity in the heart and its effects on energy transfer. A. ATP (Energy) production. The metabolism of fatty acid and glucose leads to the production of ATP in the mitochondria via oxidative phosphorylation. B. ATP/Energy transfer by the Phospho-Creatine (PCr) Shuttle. The PCr shuttle transfers ATP by transferring high energy phosphates on to and off of creatine through the CK isoforms mtCK and CK-M/B. CK isoforms can be compartmentalized at specific intracellular sites or can be found free in the cytosol. The CK-MM isoform has been identified in myofibrils as part of the structures localized to the M band (Wallimann and Eppenberger 1985). C. ATP utilization by ATPases. CK-M/B localized adjacent to ATPases utilize ATP to perform work, such as sarcomere/muscle contraction, electromechanical coupling, and action potentials. The CK-MM is functionally coupled to the myosin ATPase, allowing the efficient maintenance of high ATP/ADP locally, stimulating ATPase activity and ensuring proper contraction of the muscle (Ventura-Clapier et al. 1994). CK-MM is also bound to the membranes of the sarcoplasmic reticulum, where it is coupled functionally to Ca2+-ATPase (Minajeva et al. 1996;Rossi et al. 1990), ensuring efficient energy provision of local generation of ATP. A smaller amount of CK-MM is associated with Na+/K+-ATPase (Wallimann et al. 1989). D. The ubiquitin proteasome system (UPS) in protein quality control. The UPS, including E1, E2, and E3’s such as MuRF1, interact and ubiquitinate CK, likely targeting the substrates for degradation in a dynamic process of protein quality control. Adapted from: Zhou 2005 (Zhao et al. 2007) and Willis (Willis and Patterson 2006).
The ubiquitin ligase MuRF1 regulates CK protein quality control and activity
MuRF family proteins are mechanically responsive proteins that have been implicated in various aspect of contractile regulation and myogenic responses (Hoshijima 2006). MuRF family members have RING finger motifs that have demonstrated ubiquitin ligase (E3) activity targeted to a number of adjacent sarcomere proteins including troponin I, myosin binding protein and myosin binding protein-C (Kedar et al. 2004). More recently, a yeast two-hybrid screening of skeletal muscle cDNA libraries using full length human MuRF1 as bait revealed that MuRF-1 interacts with muscle enzymes involved with metabolism such as muscle CK and adenylate kinase 1, both key regulators of ATP transfer in the cell (Witt et al. 2005). The results of these studies suggested that MuRF1 might modulate metabolism by directly interacting with key regulatory enzymes. This hypothesis paved the way for further investigation into the mechanism by which MuRF1 regulates cardiac function during stress.
MuRF1 preferentially ubiquitinates oxidized CK
In vitro ubiquitination assays led to the discovery that MuRF1 has the ability to ubiquitinate CK (Zhao et al. 2007). More importantly though, this reaction is enhanced in the setting of oxidation, suggesting that the cell preferentially targets the oxidized form of CK-MM for degradation. This is a fascinating finding and hints at a possible quality control mechanism within the cell. In order to target the regeneration of ATP to the area in most need of this energy supply, CK-MM specifically binds to the M-line of the sarcomere via an interaction with myomesin. When the ability of wild-type versus oxidized CK-MM to bind to myomesin was investigated, it was discovered that once CK-MM is oxidized it loses it ability to integrate into the M-line. Since the M-line-bound CK-MM is crucial for ATP production, particularly during times of stress, one conclusion for the preferred ubiquitination of oxidized CK-MM is that this isoform is targeted for degradation so as to increase the amount of functional CK-MM in the cell.
Chronic ectopic expression of high levels of cardiac MuRF1 increases susceptibility to heart failure in response to pressure overload in vivo
Several lines of evidence suggest that MuRF1 acts to inhibit cardiac hypertrophy (Arya et al. 2004;Willis et al. 2007). We created transgenic mice constitutively expressing increased cardiac levels of MuRF1 (MuRF1 Tg+ mice) to further elucidate the possible underlying mechanisms of MuRF1’s regulation of cardiac hypertrophy (Willis et al. 2009b). MuRF1 Tg+ and wild-type mice appear to have little or no functional differences by catheter analysis in vivo. Surprisingly though, the increased expression of cardiac MuRF1 in the MuRF1 Tg+ mice does not save these mice from developing cardiac hypertrophy in response to trans-aortic cuffing (TAC; Willis et al. 2009b). In fact, these mice develop an eccentric pattern of hypertrophy that rapidly progresses to heart failure within 4 weeks following TAC with thinning of the anterior and posterior walls, dilation of the left ventricle and a 70% decrease in fractional shortening compared to wild-type mice (Willis et al. 2009b). These results were surprising and indicated that MuRF1’s regulation of cardiac hypertrophy is likely to be multifactorial in nature with and possibly numerous biochemical mechanisms involved.
MuRF1 regulates cardiac CK in vivo
The rapid failure of MuRF1 Tg+ mouse hearts in response to pressure overload from TAC, along with the data demonstrating that MuRF1 interacts with CK and ubiquitinates it in vitro(Koyama et al. 2008;Witt et al. 2005;Zhao et al. 2007), suggested that MuRF1’s involvement in cardiac regulation may be tied to the metabolic pathways involving CK. This hypothesis was supported by the finding that MuRF1 Tg+ mouse hearts exhibit a ~20–25% decrease in total CK activity in vitro compared to wild-type animals, both at baseline and after TAC. Despite this decrease in CK activity, the level of total ATP in MuRF1 Tg+ hearts does not significantly differ from wild type mice at baseline or after TAC. Likewise, western blot analysis of the levels of the various CK isoforms (CK-M, CK-B and CK-Mt) in MuRF1 Tg+ or wild-type mouse hearts reveals no differences at baseline or after 4 weeks TAC, suggesting that MuRF1 does not affect the steady state levels of CK.
Interestingly though, despite not seeing changes in steady state cardiac CK levels, MuRF1 Tg+ mouse hearts do exhibit evidence of enhanced post-translational modification of CK-M/B entities (Willis et al. 2009b). Recent studies have demonstrated that post-translational modification of CK and adenylate kinase 1 can occur with S-nitrosylation (addition of a NO group), leading to inhibition of activity by possible upstream signaling through NO (Shi et al. 2008). CK activity has also been shown to be regulated by reversible phosphorylation in the heart and skeletal muscle (Dieni and Storey 2009). Therefore, it is possible that MuRF1’s interaction with CK results in the post-translational modifications seen, thereby accounting for the decreased CK activity in MuRF1 Tg+ mouse hearts. This novel role for MuRF1 would be in line with the fact that the UPS is instrumental in regulating protein activity in addition to its role in targeting proteins for degradation (Kedar et al. 2004). It could also offer an explanation for why there is no heightened CK degradation in the presence of increased MuRF1 expression in the MuRF1 Tg+ mouse hearts, even though there is evidence that MuRF1 ubiquitinates CK in vitro(Koyama et al. 2008;Zhao et al. 2007). An alternative explanation for the lack of CK degradation in these mice is an increase in de-ubiquitinating activity in compensation for the constitutive MuRF1 expression in the MuRF1 Tg + heart (Figure 2D), a proposition that has yet to be tested.
While studies of MuRF1 expression in the heart using the MuRF1 Tg+ animals have been helpful in determining the downstream functions MuRF1 regulates, there are some weaknesses to this approach. First, it is not clear how physiologically relevant the chronic 45-fold increase in cardiac MuRF1 expression that is found in these mice is (Willis et al. 2009b). To date, the largest in vivo increase in MuRF1 protein expression is only 2-fold and has been reported to occur in response to ischemia or LVAD implantation (Adams et al. 2008;Willis et al. 2009a). Furthermore, the increases in MuRF1 expression that have been reported have been transient, not constitutive in nature, as in the case of the MuRF1 Tg+ mice (Adams et al. 2008). Complicating the picture more is the debate of the role of CK in heart failure. CK-deficient mice are prone to develop heart failure when challenged with increased workloads (Boehm et al. 2000) but appear normal at baseline, quite similar to the MuRF1 Tg+ mice (Willis et al. 2009b). While reduced CK activity contributes to impaired contractile reserves (Ingwall 2009), it is not necessarily entirely responsible for the decreased contractility found in heart failure. For example, other key phosphotransferase systems and enzymatic regulation of substrate utilization also appear to be affected (Dzeja et al. 2000;Ingwall 2009). Similarly, the increased susceptibility of MuRF1 Tg+ hearts to heart failure in response to pressure overload may not be caused directly through MuRF1’s effects on CK. Chronic increase in cardiac MuRF1 does result in significant changes in genes regulating oxidative phosphorylation (such as PGC-1) and the TCA cycle at the mRNA level (Willis et al. 2009b). Similarly, mice lacking MuRF1 undergo an exaggerated pathological cardiac hypertrophy in response to TAC, suggesting an essential need for MuRF1 in normal cardiac regulation. Therefore, the balance between a healthy heart, cardiac hypertrophy, and heart failure may come down to a tightly regulated titration of MuRF1 protein expression. With this in mind, it may be possible to therapeutically regulate MuRF1 levels to target critical metabolic functions. However, these ideas have yet to be tested.
Acknowledgments
Funding: The authors are supported by the American Heart Associations (Scientist Development Grant to M.S.W.) and the National Heart, Lung, and Blood Institute grant R01HL065619 (to C.P).
Glossary
- AMPK
AMP-activated protein kinase
- CK
creatine kinase
- MuRF1
Muscle Ring Finger-1
- CK/PCr
Creatine kinase/Phospho-Creatine
- CK-BB
creatine kinase isoform with two B subunits
- CK-MB
creatine kinase isoform with M and B subunits
- CK-MM
creatine kinase isoform with two M subunits
- CK-M/B
creatine kinase isoform with M and/or B subunits
- E1
ubiquitin activating enzyme
- E2
ubiquitin conjugating enzyme
- E3
ubiquitin ligase
- mtCK
mitochondrial creatine kinase isoform
- MuRF1 Tg+
MuRF1 transgenic
- UPS
ubiquitin proteasome system
Footnotes
Disclosures: The authors do not have any potential conflicts of interest relevant to this article to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams V, Linke A, Gielen S, et al. Modulation of Murf-1 and MAFbx expression in the myocardium by physical exercise training. Eur J Cardiovasc Prev Rehabil. 2008;15:293–299. doi: 10.1097/HJR.0b013e3282f3ec43. [DOI] [PubMed] [Google Scholar]
- Arya R, Kedar V, Hwang JR, et al. Muscle ring finger protein-1 inhibits PKC{epsilon} activation and prevents cardiomyocyte hypertrophy. J Cell Biol. 2004;167:1147–1159. doi: 10.1083/jcb.200402033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm E, Ventura-Clapier R, Mateo P, et al. Glycolysis supports calcium uptake by the sarcoplasmic reticulum in skinned ventricular fibres of mice deficient in mitochondrial and cytosolic creatine kinase. J Mol Cell Cardiol. 2000;32:891–902. doi: 10.1006/jmcc.2000.1130. [DOI] [PubMed] [Google Scholar]
- Dieni CA, Storey KB. Creatine kinase regulation by reversible phosphorylation in frog muscle. Comp Biochem Physiol B Biochem Mol Biol. 2009;152:405–412. doi: 10.1016/j.cbpb.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Dunlop RA, Brunk UT, Rodgers KJ. Oxidized proteins: Mechanisms of removal and consequences of accumulation. IUBMB Life. 2009;61:522–527. doi: 10.1002/iub.189. [DOI] [PubMed] [Google Scholar]
- Dzeja PP, Redfield MM, Burnett JC, et al. Failing energetics in failing hearts. Curr Cardiol Rep. 2000;2:212–217. doi: 10.1007/s11886-000-0071-9. [DOI] [PubMed] [Google Scholar]
- Elsasser S, Chandler-Militello D, Muller B, et al. Rad23 and Rpn10 serve as alternative ubiquitin receptors for the proteasome. J Biol Chem. 2004;279:26817–26822. doi: 10.1074/jbc.M404020200. [DOI] [PubMed] [Google Scholar]
- Hoshijima M. Mechanical stress-strain sensors embedded in cardiac cytoskeleton: Z disk, titin, and associated structures. Am J Physiol Heart Circ Physiol. 2006;290:H1313–1325. doi: 10.1152/ajpheart.00816.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingwall JS. Energy metabolism in heart failure and remodelling. Cardiovasc Res. 2009;81:412–419. doi: 10.1093/cvr/cvn301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakkola P, Mole DR, Tian YM, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- Kedar V, McDonough H, Arya R, et al. Muscle-specific RING finger 1 is a bona fide ubiquitin ligase that degrades cardiac troponin I. Proc Natl Acad Sci U S A. 2004;101:18135–18140. doi: 10.1073/pnas.0404341102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S, Hata S, Witt CC, et al. Muscle RING-finger protein-1 (MuRF1) as a connector of muscle energy metabolism and protein synthesis. J Mol Biol. 2008;376:1224–1236. doi: 10.1016/j.jmb.2007.11.049. [DOI] [PubMed] [Google Scholar]
- Li HH, Willis MS, Lockyer P, et al. Atrogin-1 inhibits Akt-dependent cardiac hypertrophy in mice via ubiquitin-dependent coactivation of Forkhead proteins. J Clin Invest. 2007;117:3211–3223. doi: 10.1172/JCI31757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minajeva A, Ventura-Clapier R, Veksler V. Ca2+ uptake by cardiac sarcoplasmic reticulum ATPase in situ strongly depends on bound creatine kinase. Pflugers Arch. 1996;432:904–912. doi: 10.1007/s004240050214. [DOI] [PubMed] [Google Scholar]
- Pines J. Mitosis: a matter of getting rid of the right protein at the right time. Trends Cell Biol. 2006;16:55–63. doi: 10.1016/j.tcb.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Richly H, Rape M, Braun S, et al. A series of ubiquitin binding factors connects CDC48/p97 to substrate multiubiquitylation and proteasomal targeting. Cell. 2005;120:73–84. doi: 10.1016/j.cell.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Rossi AM, Eppenberger HM, Volpe P, et al. Muscle-type MM creatine kinase is specifically bound to sarcoplasmic reticulum and can support Ca2+ uptake and regulate local ATP/ADP ratios. J Biol Chem. 1990;265:5258–5266. [PubMed] [Google Scholar]
- Shi Q, Feng J, Qu H, et al. A proteomic study of S-nitrosylation in the rat cardiac proteins in vitro. Biol Pharm Bull. 2008;31:1536–1540. doi: 10.1248/bpb.31.1536. [DOI] [PubMed] [Google Scholar]
- Ventura-Clapier R, Veksler V, Hoerter JA. Myofibrillar creatine kinase and cardiac contraction. Mol Cell Biochem. 1994;133–134:125–144. doi: 10.1007/BF01267952. [DOI] [PubMed] [Google Scholar]
- Wallimann T, Eppenberger HM. Localization and function of M-line-bound creatine kinase. M-band model and creatine phosphate shuttle. Cell Muscle Motil. 1985;6:239–285. doi: 10.1007/978-1-4757-4723-2_8. [DOI] [PubMed] [Google Scholar]
- Wallimann T, Schnyder T, Schlegel J, et al. Subcellular compartmentation of creatine kinase isoenzymes, regulation of CK and octameric structure of mitochondrial CK: important aspects of the phosphoryl-creatine circuit. Prog Clin Biol Res. 1989;315:159–176. [PubMed] [Google Scholar]
- Willis MS, Ike C, Li L, et al. Muscle ring finger 1, but not muscle ring finger 2, regulates cardiac hypertrophy in vivo. Circ Res. 2007;100:456–459. doi: 10.1161/01.RES.0000259559.48597.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis MS, Patterson C. Into the heart: the emerging role of the ubiquitin-proteasome system. J Mol Cell Cardiol. 2006;41:567–579. doi: 10.1016/j.yjmcc.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Willis MS, Rojas M, Li L, et al. Muscle ring finger 1 mediates cardiac atrophy in vivo. Am J Physiol Heart Circ Physiol. 2009a;296:H997–H1006. doi: 10.1152/ajpheart.00660.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis MS, Schisler JC, Li L, et al. Cardiac muscle ring finger-1 increases susceptibility to heart failure in vivo. Circ Res. 2009b;105:80–88. doi: 10.1161/CIRCRESAHA.109.194928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt SH, Granzier H, Witt CC, et al. MURF-1 and MURF-2 target a specific subset of myofibrillar proteins redundantly: towards understanding MURF-dependent muscle ubiquitination. J Mol Biol. 2005;350:713–722. doi: 10.1016/j.jmb.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Zhao TJ, Yan YB, Liu Y, et al. The generation of the oxidized form of creatine kinase is a negative regulation on muscle creatine kinase. J Biol Chem. 2007;282:12022–12029. doi: 10.1074/jbc.M610363200. [DOI] [PubMed] [Google Scholar]