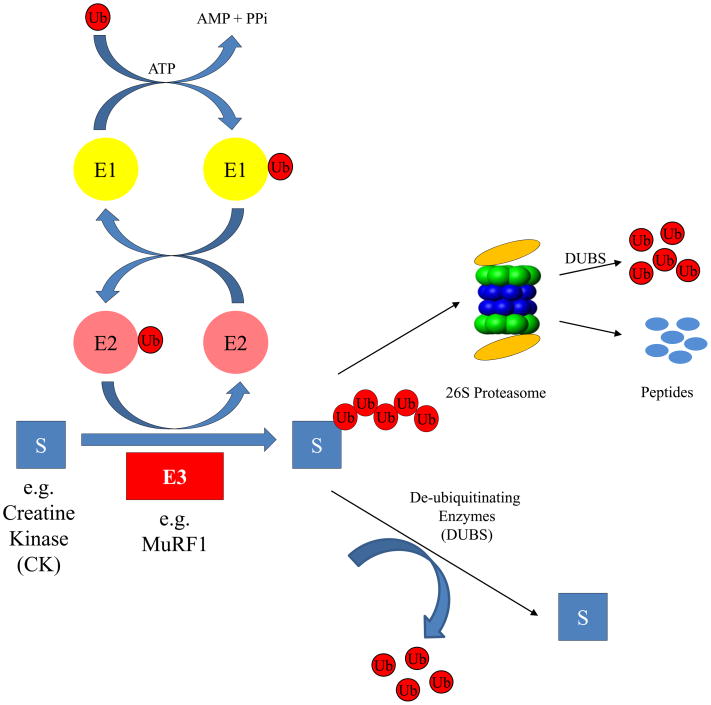
Figure 1.
The canonical Ubiquitin Proteasome System (UPS). Protein ubiquitination is driven by the action of 3 successive enzymes. The ubiquitin-activating enzyme (E1) activates ubiquitin in an ATP-dependent manner, adding the ubiquitin to the subsequent ubiquitin conjugating (E2) enzyme. Ubiquitin is then transferred from the ubiquitin-charged E2 to the substrate and/or the growing ubiquitin chain by the activity of a ubiquitin ligase (E3), which gives the system specificity. The multi-ubiquitin chain attached to the substrate is recognized by the 26S proteasome, which degrades the substrate and ubiquitin chain. The selective nature of the ubiquitin ligases is the focus of many investigations since it drives the specificity of the reaction. The canonical UPS degradation system shown here represents a skeletal schematic for a much more complex system, with ancillary factors participating in substrate recognition and a pathway involved in regulating protein activities by post-translational modifications that don’t always lead to degradation (Elsasser et al. 2004;Li et al. 2007;Richly et al. 2005).