Figure 1.
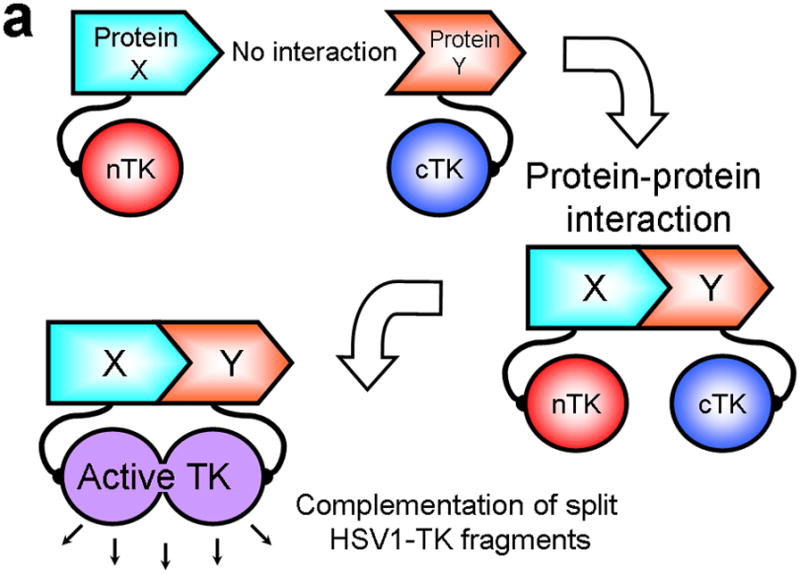
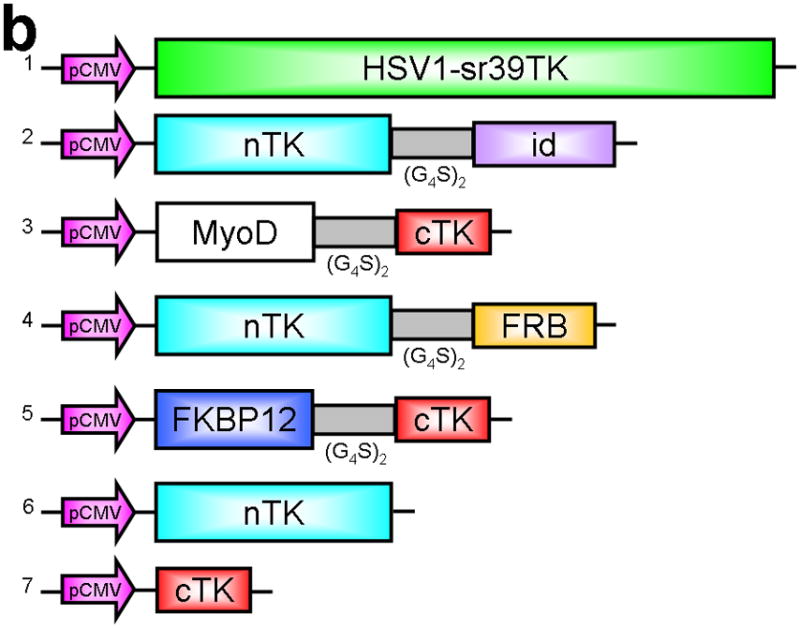
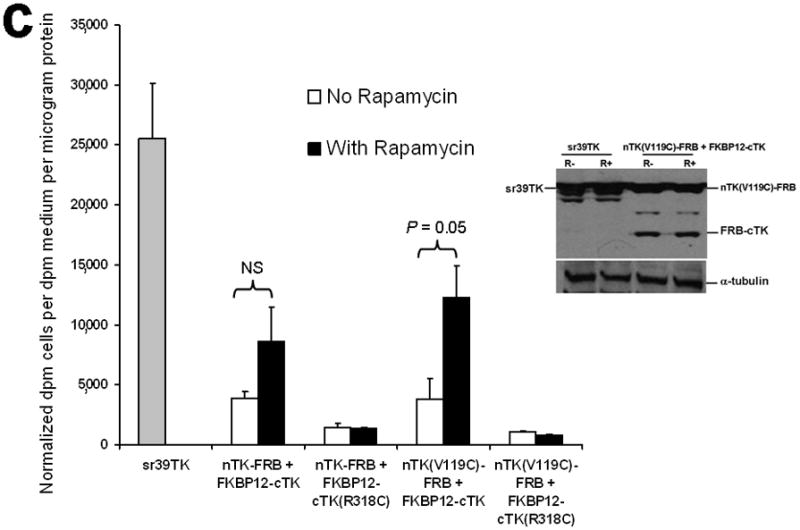
Principle of the TK PCA, expression vectors used, and evaluation of plasmid vector constructs containing the V119C mutation. (a) Schematic diagram showing the PCA strategy using split HSV1-TK (here abbreviated as TK) to monitor the hypothetical X–Y heterodimeric protein-protein interaction. This is accomplished by fusing each of the reporter fragments to heterologous X–Y protein domains to generate two chimeric proteins that have the capacity to interact with one another. If the interaction of the two heterologous protein domains (first and foremost) restores the activity of the reporter by bringing the two reporter fragments into close spatial proximity (as a secondary consequence), then this restoration of reporter activity can be used to monitor the interaction of the two heterologous protein domains. Dimerization of the two proteins restores TK activity through protein complementation and produces a PET imaging signal in the presence of radiolabeled TK substrate. If the reporter protein is an enzyme, then an additional strength of this PCA approach is the capacity to amplify the signal associated with each protein-protein interaction event. Note that this is a simplified schematic diagram representing the forward or ‘folding’ mechanism underlying a PCA. The reverse or ‘unfolding’ mechanism is not depicted here for the sake of simplification. A more realistic and detailed graphic depiction of the interplay between these two mechanisms within a PCA has been published previously by Michnick et al.26. (b) Schematic representation of the plasmid vector constructs made for transient expression of the seven genes transfected individually or in combinations described in the text and Supplementary Methods, for evaluation of the PCA strategy. Each vector was cloned into a pcDNA3.1 (+) plasmid backbone, under control of a CMV promoter. (c) Graph to show comparison of coexpressed chimeras carrying nTK or cTK with FRB/FKBP12 (with and without rapamycin), and chimeras containing the TK point mutations V119C and R318C on enzyme activity in a PCA, measured by TK enzyme uptake (expressed as normalized dpm of cells/dpm in medium/microgram of protein) in transiently transfected 293T cells, with mock (negative) and full length HSV1-sr39TK (positive) controls. The error bar is the standard error of the mean for three samples. Introducing the point mutation V119C to the nTK fragment resulted in an increase (at limit of statistical significance) in measured TK activity upon addition of rapamycin, after co-transfecting nTK(V119C)-FRB and FKBP12-cTK. NS: not significant. Western blot analysis using antibody to TK in the presence or absence of Rapamycin shows adequate expression levels in 293T cells.
